Question:
Emf of the following cell at $298 \mathrm{~K}$ in $\mathrm{V}$ is $x \times 10^{-2} . \mathrm{Zn}\left|\mathrm{Zn}^{2+}(0.1 \mathrm{M}) \| \mathrm{Ag}^{+}(0.01 \mathrm{M})\right| \mathrm{Ag}$ The value of $x$ is________. (Rounded off to the nearest integer)
$\left[\right.$ Given $\left.: \mathrm{E}_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{0}=-0.76 \mathrm{~V} ; \mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{0}=+0.80 \mathrm{~V} ; \frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.059\right]$
Solution:
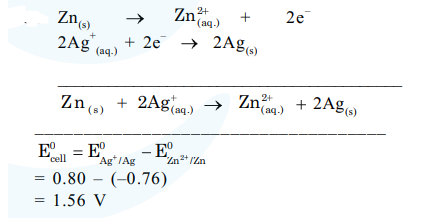
$\mathrm{E}_{\text {cell }}=1.56 \frac{-0.059}{2} \log \frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Ag}^{+}\right]^{2}}$
$=1.56-\frac{0.059}{2} \log \frac{0.1}{(0.01)^{2}}$
$=1.56-\frac{0.059}{2} \times 3$
$=1.56-0.0885$
$=1.4715$
$=147.15 \times 10^{-2}$
