Question.
Draw the electron dot structure of
(a) Ethanoic acid
(b) $\mathrm{H}_{2} \mathrm{~S}$ (Hydrogen sulphide)
(c) Propanone
(d) $\mathrm{F}_{2}$ (Fluorine)
Draw the electron dot structure of
(a) Ethanoic acid
(b) $\mathrm{H}_{2} \mathrm{~S}$ (Hydrogen sulphide)
(c) Propanone
(d) $\mathrm{F}_{2}$ (Fluorine)
solution:
(a) Ethanoic acid
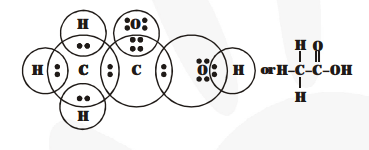
(b) $\mathrm{H}_{2} \mathrm{~S}$ (Hydrogen sulphide)
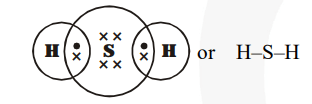
(c) Propanone
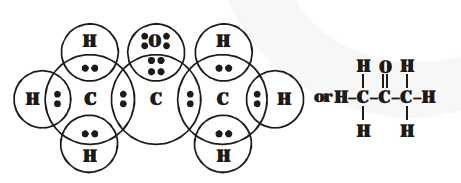
(d) $\mathrm{F}_{2}$ (Fluorine)
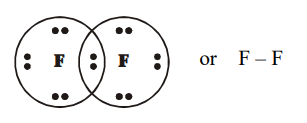
(a) Ethanoic acid
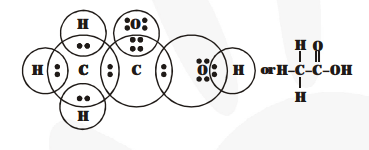
(b) $\mathrm{H}_{2} \mathrm{~S}$ (Hydrogen sulphide)
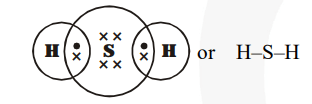
(c) Propanone

(d) $\mathrm{F}_{2}$ (Fluorine)

