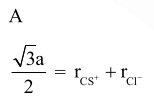Question: CsCl crystallises in body centred cubic lattice. if 'a' is its edge length then which of the
following expression is correct :
$\mathrm{r}_{\mathrm{Cs}^{+}}+\mathrm{r}_{\mathrm{Cl}^{-}}=\frac{\sqrt{3}}{2} \mathrm{a}$
$\mathrm{r}_{\mathrm{cs}^{+}}+\mathrm{r}_{\mathrm{Cl}^{-}}=\sqrt{3 \mathrm{a}}$
$\mathrm{r}_{\mathrm{Cs}^{+}}+\mathrm{r}_{\mathrm{Cl}^{-}}=3 \mathrm{a}$
$\mathrm{r}_{\mathrm{Cs}^{+}}+\mathrm{r}_{\mathrm{Cl}^{-}}=\frac{3 \mathrm{a}}{2}$
Correct Option: 1
Solution: