Question:
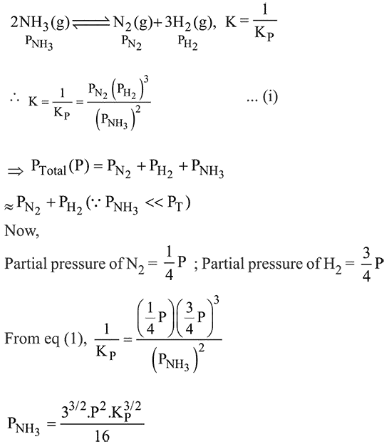
Consider the reaction $\mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NH}_{3}(\mathrm{~g})$
The equilibrium constant of the above reaction is $\mathrm{K}_{\mathrm{P}}$.If pure ammonia is left to dissociate, the partial pressure of ammonia at equilibrium is given by (Assume that $\mathrm{P}_{\mathrm{NH}_{3}}<<\mathrm{P}_{\text {total }}$ at equilibrium $)$
Correct Option: 1
Solution: