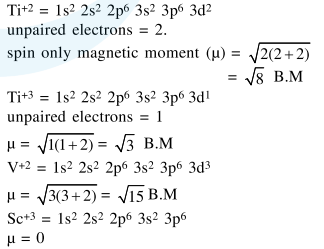
Question: Consider the hydrates ions of $\mathrm{Ti}^{2+}, \mathrm{V}^{2+}, \mathrm{Ti}^{3+}$ and $\mathrm{Sc}^{3+}$. The correct order of their spin-only magnetic moments is :
$\mathrm{Sc}^{3+}<\mathrm{Ti}^{3+}<\mathrm{Ti}^{2+}<\mathrm{V}^{2+}$
$\mathrm{Ti}^{3+}<\mathrm{Ti}^{2+}<\mathrm{Sc}^{3+}<\mathrm{V}^{2+}$
$\mathrm{Sc}^{3+}<\mathrm{Ti}^{3+}<\mathrm{V}^{2+}<\mathrm{Ti}^{2+}$
$\mathrm{V}^{2+}<\mathrm{Ti}^{2+}<\mathrm{Ti}^{3+}<\mathrm{Sc}^{3+}$
Correct Option: 1
Solution: