Question:
Consider the following statements
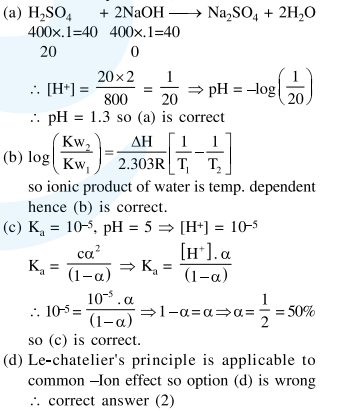
(a) The $\mathrm{pH}$ of a mixture containing $400 \mathrm{~mL}$ of $0.1 \mathrm{M} \mathrm{H}_{2} \mathrm{SO}_{4}$ and $400 \mathrm{~mL}$ of $0.1 \mathrm{M} \mathrm{NaOH}$ will be approximately 1.3.
(b) Ionic product of water is temperature dependent.
(c) A monobasic acid with $\mathrm{K}_{\mathrm{a}}=10^{-5}$ has a $\mathrm{pH}=5$. The degree of dissociation of this acid is $50 \%$.
(d) The Le Chatelier's principle is not applicable to common-ion effect.
the correct statement are :
Correct Option: , 2
Solution: