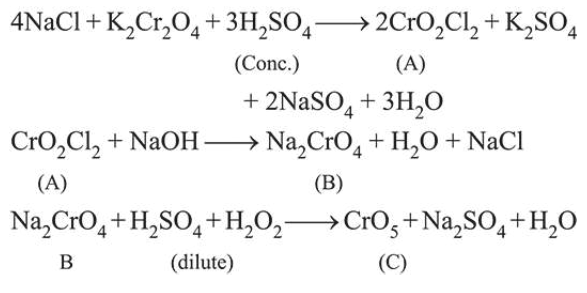
Question:
Consider the following reactions:
$\mathrm{NaCl}+\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}+\mathrm{H}_{2} \mathrm{SO}_{4} \rightarrow(\mathrm{A})+$ Side products (Conc.)
$(A)+\mathrm{NaOH} \rightarrow(\mathrm{B})+$ Side products
(B) $+\mathrm{H}_{2} \mathrm{SO}_{4}+\mathrm{H}_{2} \mathrm{O}_{2} \rightarrow$ (C) $+$ Side products (dilute)
The sum of the total number of atoms in one molecule each of $(A),(B)$ and $(C)$ is ______________ .
Solution:
(18.00)