Question:
Consider the following molecules and statements related to them :
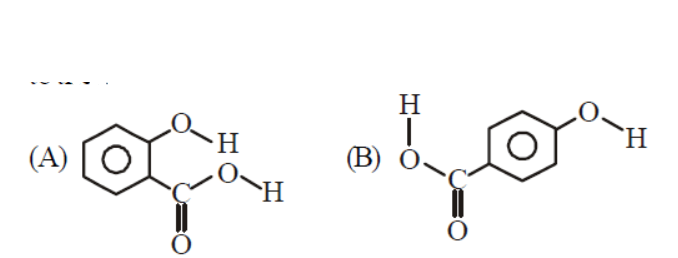
(a) (B) is more likely to be crystalline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below :
Correct Option: , 4
Solution:
Molecule (A) shows intramolecular H-bonding while molecule (B) shows intermolecular H-bonding. Due to presence of intermolecular H-bonding it has more b. pt. than molecule (A). Molecule (B) also shows intermolecule H-bonding with water which makes it more soluble than A.
(B) is crystalline solid while (A) is liquid at room tempertature because of weaker intramolecular hydrogen bonding.
