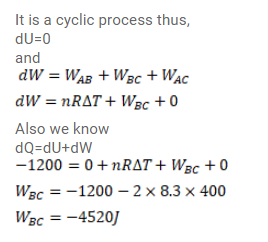Question:
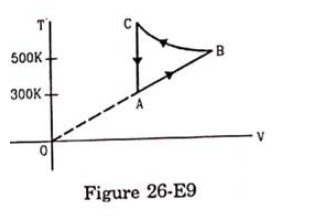
Consider the cyclic process ABCA, shown in figure (26-E9), performed on a sample of $2.0$ mole of an ideal gas. A total of $1200 \mathrm{~J}$ of heat is withdrawn from the sample in the process. Find the work done by the gas during the part $B C$.
Solution: