Compare the relative stability of the following species and indicate their magnetic properties;
$\mathrm{O}_{2}, \mathrm{O}_{2}^{+}, \mathrm{O}_{2}^{-}$(superoxide), $\mathrm{O}_{2}^{2-}$ (peroxide)
There are 16 electrons in a molecule of dioxygen, 8 from each oxygen atom. The electronic configuration of oxygen molecule can be written as:
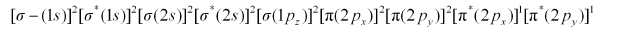
Since the 1s orbital of each oxygen atom is not involved in boding, the number of bonding electrons = 8 = Nb and the number of anti-bonding orbitals = 4 = Na.
Bond order $=\frac{1}{2}\left(N_{\mathrm{b}}-N_{\mathrm{a}}\right)$
$=\frac{1}{2}(8-4)$
$=2$
Similarly, the electronic configuration of $\mathrm{O}_{2}^{+}$can be written as:
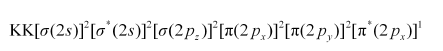
Nb = 8
Na = 3
Bond order of $\mathrm{O}_{2}^{+}=\frac{1}{2}(8-3)$
= 2.5
Electronic configuration of $\mathrm{O}_{2}^{-}$ion will be:
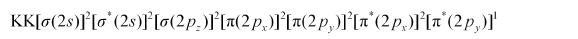
Nb = 8
Na = 5
Bond order of $\mathrm{O}_{2}^{-}=\frac{1}{2}(8-5)$
= 1.5
Electronic configuration of $\mathrm{O}_{2}^{2-}$ ion will be:
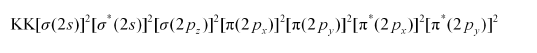
Nb = 8
Na = 6
Bond order of $\mathrm{O}_{2}^{2-}=\frac{1}{2}(8-6)$
= 1
Bond dissociation energy is directly proportional to bond order. Thus, the higher the bond order, the greater will be the stability. On this basis, the order of stability is
$\mathrm{O}_{2}^{+}>\mathrm{O}_{2}>\mathrm{O}_{2}^{-}>\mathrm{O}_{2}^{2-}$
