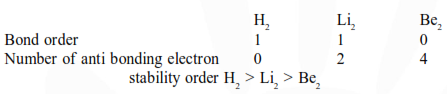Question: Bond order normally gives idea of stability of a molecular species. All the molecules viz. $\mathrm{H}_{2}$, $\mathrm{Li}_{2}$ and $\mathrm{B}_{2}$ have the same bond order yet they are not equally stable. Their stability order is:
$\mathrm{Li}_{2}>\mathrm{H}_{2}>\mathrm{B}_{2}$
$\mathrm{H}_{2}>\mathrm{B}_{2}>\mathrm{Li}_{2}$
$\mathrm{B}_{2}>\mathrm{H}_{2}>\mathrm{Li}_{2}$
$\mathrm{Li}_{2}>\mathrm{B}_{2}>\mathrm{H}_{2}$
Correct Option: , 2
Solution: