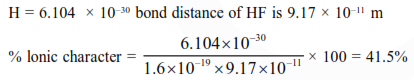Question: Bond distance in HF is $9.17 \times 10^{-11} \mathrm{~m}$. Dipole moment of $\mathrm{HF}$ is $6.104 \times 10^{-30} \mathrm{Cm}$. The percent ionic character in HF will be : (electron charge $=1.60 \times 10^{-19} \mathrm{C}$ )
$61.0 \%$
$38.0 \%$
$35.5 \%$
$41.5 \%$
Correct Option: , 4
Solution: