Question:
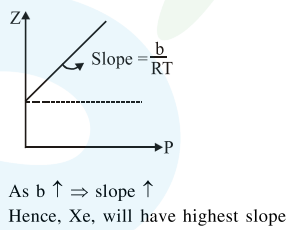
At a given temperature $\mathrm{T}$, gases Ne, Ar, Xe and $\mathrm{Kr}$ are found to deviate from ideal gas behaviour. Their equation of state is given as
$\mathrm{p}=\frac{\mathrm{RT}}{\mathrm{V}-\mathrm{b}}$ at $\mathrm{T}$
Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of $\mathrm{Z}$ (compression factor) vs p?
Correct Option: , 3
Solution: