Question:
Assuming that $\mathrm{Ba}(\mathrm{OH})_{2}$ is completely ionised in aqueous solution under the given conditions the concentration of $\mathrm{H}_{3} \mathrm{O}^{+}$ions in $0.005 \mathrm{M}$ aqueous solution of $\mathrm{Ba}(\mathrm{OH})_{2}$ at $298 \mathrm{~K}$ is___________$\times 10^{-12}$ mol $\mathrm{L}^{-1}$. (Nearest integer)
Solution:
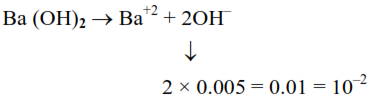
At $298 \mathrm{~K}$ : in aq. solution $\left[\mathrm{H}_{3} \mathrm{O}^{+}\right][\mathrm{OH}]=10^{-14}$
$\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\frac{10^{-14}}{10^{-2}}=10^{-12}$
