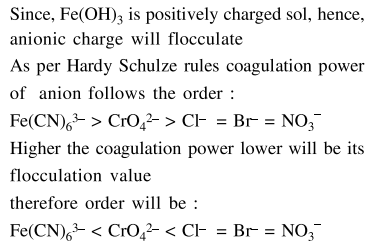
Question: As per Hardy-Schulze formulation, the flocculation values of the following for ferric hydroxide sol are in the order:
$\mathrm{AlCl}_{3}>\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]>\mathrm{K}_{2} \mathrm{CrO}_{4}>\mathrm{KBr}=\mathrm{KNO}_{3}$
$\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]<\mathrm{K}_{2} \mathrm{CrO}_{4}<\mathrm{AlCl}_{3}<\mathrm{KBr}<\mathrm{KNO}_{3}$
$\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]>\mathrm{AlCl}_{3}>\mathrm{K}_{2} \mathrm{CrO}_{4}>\mathrm{KBr}>\mathrm{KNO}_{3}$
$\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]<\mathrm{K}_{2} \mathrm{CrO}_{4}<\mathrm{KBr}=\mathrm{KNO}_{3}=\mathrm{AlCl}_{3}$
Correct Option: , 4
Solution: