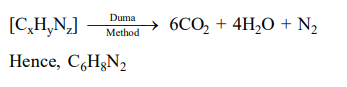
Question: An organic compound is estimated through Dumus method and was found to evolve 6 moles of $\mathrm{CO}_{2}$. 4 moles of $\mathrm{H}_{2} \mathrm{O}$ and 1 mole of nitrogen gas. The formula of the compound is :
$\mathrm{C}_{12} \mathrm{H}_{8} \mathrm{~N}$
$\mathrm{C}_{12} \mathrm{H}_{8} \mathrm{~N}_{2}$
$\mathrm{C}_{6} \mathrm{H}_{8} \mathrm{~N}$
$\mathrm{C}_{6} \mathrm{H}_{8} \mathrm{~N}_{2}$
Correct Option: , 4
Solution: