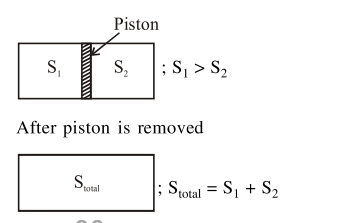
Question:
An ideal gas in a cylinder is separated by a piston in such a way that the entropy of one part is $S_{1}$ and that of the other part is $S_{2}$. Given that $\mathrm{S}_{1}>\mathrm{S}_{2}$. If the piston is removed then the total entropy of the system will be :
Correct Option: , 4
Solution: