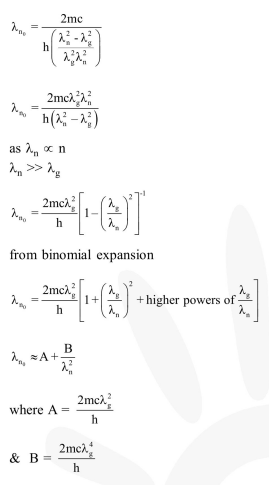
Question:
An electron from various excited states of hydrogen atom emit radiation to come to the ground state. Let $\lambda_{\mathrm{n}}, \lambda_{\mathrm{g}}$ be the de Broglie wavelength of the electron in the $\mathrm{n}^{\text {th }}$ state and the ground state respectively. Let $\Lambda_{n}$ be the wavelength of the emitted photon in the transition from the $n^{\text {th }}$ state to the ground state. For large $n,(A, B$ are constants)
Correct Option: , 4
Solution: