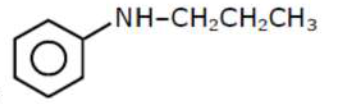An amine on reaction with benzenesulphonyl chloride produces a compound insoluble in alkaline solution.
Question:
An amine on reaction with benzenesulphonyl chloride produces a compound insoluble in alkaline solution. This amine can be prepared by ammonolysis of ethyl chloride. The correct structure of amine is:
Correct Option:
Solution:
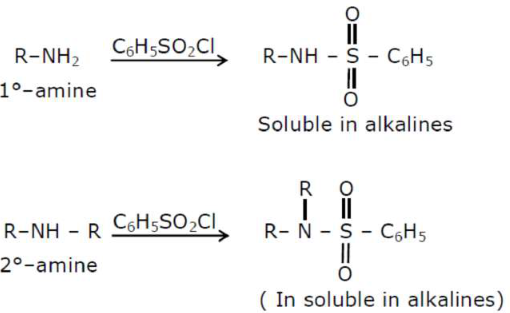
According to the question the amine should be $2^{\circ}$-amine, in which one of the alkyl group should be ethyl, because it can be formed by ammonolysis of ethyl chloride