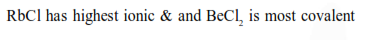Question: Amongst $\mathrm{LiCl}, \mathrm{RbCl}, \mathrm{BeCl}_{2}$ and $\mathrm{MgCl}_{2}$ the compounds with the greatest and the least ionic character, respectively are :
$\mathrm{RbCl}$ and $\mathrm{MgCl}_{2}$
$\mathrm{LiCl}$ and $\mathrm{RbCl}$
$\mathrm{MgCl}_{2}$ and $\mathrm{BeCl}_{2}$
$\mathrm{RbCl}$ and $\mathrm{BeCl}_{2}$
Correct Option: , 4
Solution: