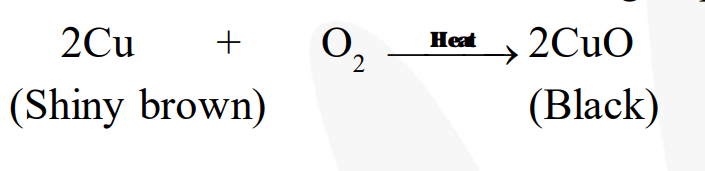Question.
A shiny brown-coloured element ' $\mathrm{X}$ ' on heating in air becomes black in colour. Name the element ' $\mathrm{X}^{\prime}$ and the black coloured compound formed.
A shiny brown-coloured element ' $\mathrm{X}$ ' on heating in air becomes black in colour. Name the element ' $\mathrm{X}^{\prime}$ and the black coloured compound formed.
solution:
$' X^{\prime}$ is copper $(\mathrm{Cu})$ and the black-coloured compound formed is copper oxide $(\mathrm{CuO})$. The equation of the reaction involved on heating copper is given below.
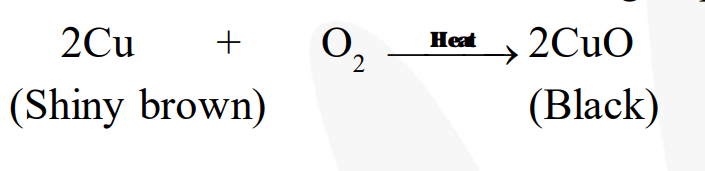
$' X^{\prime}$ is copper $(\mathrm{Cu})$ and the black-coloured compound formed is copper oxide $(\mathrm{CuO})$. The equation of the reaction involved on heating copper is given below.