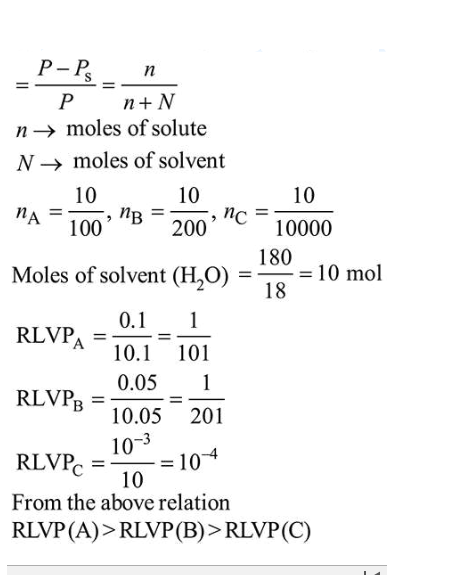
A set of solutions is prepared using 180 g of water as a solvent and 10g of different non-volatile solutes A, B and C.
Question:
A set of solutions is prepared using $180 \mathrm{~g}$ of water as a solvent and $10 \mathrm{~g}$ of different non-volatile solutes A, B and C. The relative lowering of vapour pressure in the presence of these solutes are in the order
[Given, molar mass of $\mathrm{A}=100 \mathrm{~g} \mathrm{~mol}^{-1} ; \mathrm{B}=200 \mathrm{~g} \mathrm{~mol}^{-1} ; \mathrm{C}=10,000 \mathrm{~g} \mathrm{~mol}^{-1}$ ]
Correct Option: , 3
Solution:
Relative lowering in vapour pressure (RLVP)