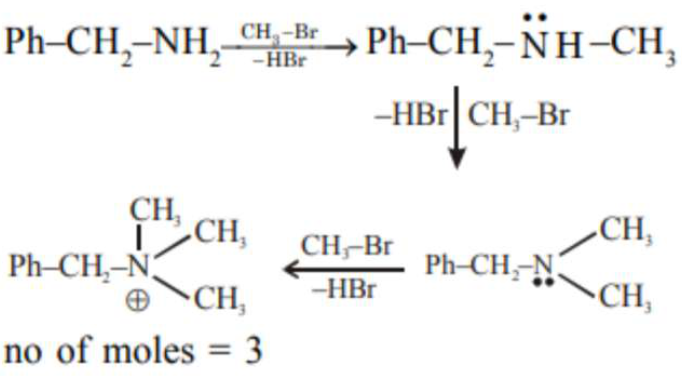
A reaction of 0.1 mole of Benzylamine with bromomethane gave 23 g of Benzyl trimethyl ammonium bromide.
Question:
A reaction of $0.1$ mole of Benzylamine with bromomethane gave $23 \mathrm{~g}$ of Benzyl trimethyl ammonium bromide. The number of moles of bromomethane consumed in this reaction are $\mathrm{n} \times 10^{-1}$, when $\mathrm{n}=\ldots$ (Round off to the Nearest Integer).
(Given : Atomic masses: C: $12.0 \mathrm{u}$, $\mathrm{H}: 1.0 \mathrm{u}, \mathrm{N}: 14.0 \mathrm{u}, \mathrm{Br}: 80.0 \mathrm{u}]$
Solution:
(3)