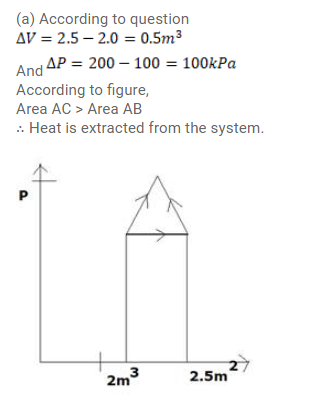
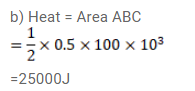Question:
A gas is initially at a pressure of $100 \mathrm{kPa}$ and its volume is $2.0 \mathrm{~m}^{3}$. Its pressure is kept constant and the volume is changed from $2.0 \mathrm{~m}^{3}$ to $2.5 \mathrm{~m}^{3}$. Its volume is now kept constant and the pressure is increased from $100 \mathrm{kPa}$ to $200 \mathrm{kPa}$. The gas is brought back to its volume.
(a) Whether the heat is supplied to or extracted from the gas in the complete cycle?
(b) How much heat was supplied or extracted?
Solution: