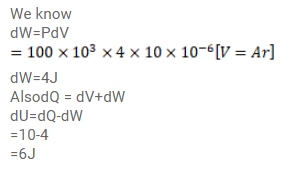Question:
A gas is enclosed in a cylindrical vessel fitted with a frictionless piston. The gas is slowly heated for some time. During the process, $10 \mathrm{~J}$ of heat is supplied and the piston is found to move out $10 \mathrm{~cm}$. Find the increase in the internal energy of the gas. The area of cross-section of the cylinder $=4 \mathrm{~cm}^{2}$ and the atmospheric pressure $=100 \mathrm{kPa}$.
Solution: