Question:
A dry pellet of a common base ‘B’, when kept in open absorbs moisture and turns sticky. The compound is also formed by chloralkali process. Identify
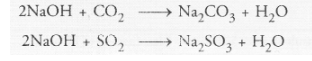
‘B’. What type of reaction occurs when B is treated with an acidic oxide ? Write a balanced chemical equation for one such solution.
Solution:
The available information suggests that the base ‘B’ is sodium hydroxide (NaOH). It is a deliquescent substance and becomes sticky on absorbing
moisture from atmosphere. It is commercially formed by the electrolysis of a strong solution of sodium chloride (brine).
It reacts with an acidic oxide such as CO2 or SO, gas to form corresponding salt and water. For example,