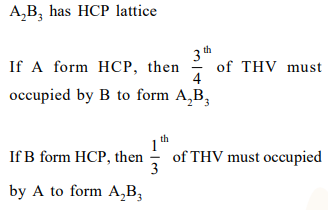
Question: A compound of formula $\mathrm{A}_{2} \mathrm{~B}_{3}$ has the hcp lattice. Which atom forms the hep lattice and what fraction of tetrahedral voids is occupied by the other atoms:
hep lattice-A, $\frac{2}{3}$ Tetrachedral voids-B
hep lattice-B, $\frac{1}{3}$ Tetrachedral voids-A
hep lattice-B, $\frac{2}{3}$ Tetrachedral voids-A
hep lattice-A $\frac{1}{3}$ Tetrachedral voids-B
Correct Option: , 2
Solution: