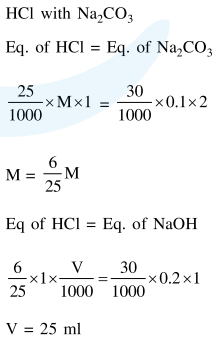Question:
$25 \mathrm{ml}$ of the given $\mathrm{HCl}$ solution requires $30 \mathrm{~mL}$ of $0.1 \mathrm{M}$ sodium carbonate solution. What is the volume of this $\mathrm{HCl}$ solution required to titrate $30 \mathrm{~mL}$ of $0.2 \mathrm{M}$ aqueous $\mathrm{NaOH}$ solution?
Correct Option: 1,
Solution: