$250 \mathrm{~mL}$ of a waste solution obtained from the workshop of a goldsmith contains $0.1 \mathrm{M} \mathrm{AgNO}_{3}$ and $0.1 \mathrm{M} \mathrm{AuCl}$. The solution was electrolyzed at $2 \mathrm{~V}$ by passing a current of 1 A for 15 minutes. The metal/metals electrodeposited will be :
$\left(\mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{0}=0.80 \mathrm{~V}, \mathrm{E}_{\mathrm{Au}^{+} / \mathrm{Au}}^{0}=1.69 \mathrm{~V}\right)$
Correct Option: 1
Millimoles of $\mathrm{Au}^{+}=0.1 \times 250=25$
Mole of $\mathrm{Au}^{+}=\frac{25}{1000}=\frac{1}{40}=0.025$
Similarly, moles of $\mathrm{Ag}^{+}=0.025$
Charge passed $=\mathrm{I} \times t=1 \times 15 \times 60=900 \mathrm{C}$
Moles of $\mathrm{e}^{-}$passed $=\frac{900}{96500}=0.0093 \mathrm{~mol}$.
Species with higher value of SRP will get deposited first at cathode.
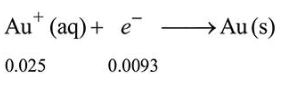
So, only Au will get deposited.
