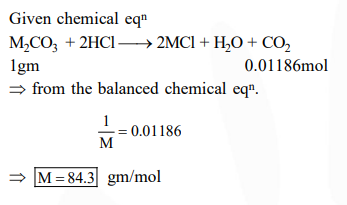
Question:
1 gram of a carbonate $\left(\mathrm{M}_{2} \mathrm{CO}_{3}\right)$ on treatment with excess $\mathrm{HCl}$ produces $0.01186$ mole of $\mathrm{CO}_{2}$. the molar mass of $\mathrm{M}_{2} \mathrm{CO}_{3}$ in $\mathrm{g} \mathrm{mol}^{-1}$ is :-
Correct Option: , 2
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
