Question:
$1.86 \mathrm{~g}$ of aniline completely reacts to form acetanilide. $10 \%$ of the product is lost during purification. Amount of acetanilide obtained after purification (in $\mathrm{g}$ ) is _________ $\times 10^{-2}$
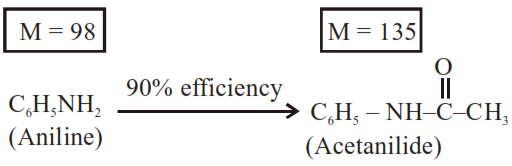
Solution:
Given $1.86 \mathrm{~g}$
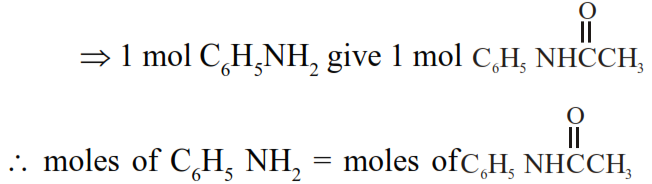
$\Rightarrow \frac{1.86}{93}=\frac{W_{\text {ace tan ilide }}}{135}$
$\Rightarrow \mathrm{W}_{\text {acelanilide }}=\frac{1.86 \times 135}{93} \mathrm{~g}=2.70 \mathrm{~g}$
But efficiency of reaction is $90 \%$ only
$\therefore$ Mass of acetanilide produced $=2.70 \times \frac{90}{100} \mathrm{~g}$
$=2.43 \mathrm{~g}$
$=243 \times 10^{-2} \mathrm{~g}$
$\Rightarrow x=243$
