
NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules PDF
Hey, are you a class 12 student and looking for ways to download NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules PDF? If yes. Then read this post till the end.In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules in PDF that are prepared by Kota’s top IITian Faculties by keeping Simplicity in mind.
If you want to learn and understand class 12 Chemistry Chapter 14 "Biomolecules" in an easy way then you can use these solutions PDF.
NCERT Solutions helps students to Practice important concepts of subjects easily. Class 12 Chemistry solutions provide detailed explanations of all the NCERT questions that students can use to clear their doubts instantly.
If you want to score high in your class 12 Chemistry Exam then it is very important for you to have a good knowledge of all the important topics, so to learn and practice those topics you can use eSaral NCERT Solutions.
In this article, we have listed NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules PDF that you can download to start your preparations anytime.
So, without wasting more time Let’s start.
Download NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules PDF
Question 1. Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Solution. Structure of glucose is:

Structure of sucrose is:

From the above structures we can see that glucose molecule contains five $-\mathrm{OH}$ groups while a sucrose molecule contains eight -OH groups. These - OHgroups are responsible for extensive hydrogen bonding with water.
Due to hydrogen bonding these two compounds are soluble in water.
Cyclohexane and benzene (simple six membered ring compounds) do not contain −OH groups. So they cannot undergo H −bonding with water and hence, are insoluble in water.
Question 2. What are the expected products of hydrolysis of lactose?
Solution. Lactose is a disaccharide carbohydrate composed of $\beta-$ D galactose and $\beta-D$ glucose units. Thus, on hydrolysis, it gives $\beta-$ D galactose and $\beta-D$ glucose due to the breaking of the glycosidic bond.


Question 3. How do you explain the absence of aldehyde group in the pentaacetate of D − glucose?
Solution. D - glucose reacts with hydroxylamine $\left(\mathrm{NH}_{2} \mathrm{OH}\right)$ to form an oxime due to the presence of aldehyde (-CHO) group or carbonyl carbon. This happens because the cyclic structure of glucose forms an open chain structure in an aqueous medium, which then reacts with $\mathrm{NH}_{2} \mathrm{OH}$ to give an oxime.

Whereas pentaacetate of $D-$ glucose does not react with $\mathrm{NH}_{2} \mathrm{OH}$ because pentaacetate does not form an open chain structure.


Question 4. The melting points and solubility in water of amino acids are generally higher than that of he corresponding halo acids. Explain.
Solution. Amino acids are compounds containing both acidic (carboxyl) as well as basic (amino) groups. In aqueous solutions, the carboxyl group can lose a proton $(\mathrm{H}+)$ and the amino group can accept a proton giving rise to a dipolar ion known as a Zwitter ion.

Due to this dipolar behaviour, there is a development of strong electrostatic interactions between the molecules and water. But halo-acids are not known to exhibit such dipolar behaviour. Hence, the melting points and the solubility of amino acids in water is higher than those of the halo-acids.
Question 5. Where does the water present in the egg go after boiling the egg?
Solution. When an egg is boiled, the proteins present inside the egg get denatured (the process of loss of biological activity of protein like hydrogen bonding when subjected to a physical change) and coagulate (the process of a liquid changing to a solid or semi-solid state). After boiling the egg, the water present in it gets absorbed by the coagulated protein through hydrogen bonding.
Question 6. Why cannot vitamin C be stored in our body?
Solution. Vitamin $\mathrm{C}$ is water soluble unlike other vitamins A, D, E and $\mathrm{K}$ which are fat soluble. As a result, it is rapidly absorbed from the intestine and readily excreted in the urine. Hence vitamin C cannot be stored in our body
Question 7. What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Solution. When a nucleotide from DNA containing thymine is hydrolyzed, thymine, $\beta-D-2-$ deoxyribose and phosphoric acid are obtained as products.
Question 8. When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Solution. A DNA molecule is double-stranded molecule (two polynucleotide chains whose nitrogenous bases are connected by hydrogen bonds) in which the pairing of bases occurs. In this molecule Adenine always pairs with thymine, while cytosine always pairs with guanine. Therefore, on hydrolysis of DNA, the quantity of adenine produced is equal to that of thymine and similarly, the quantity of cytosine is equal to that of guanine. But when RNA is hydrolyzed, there is no relationship among the quantities of the different bases obtained. This shows that RNA is a single-stranded molecule.
Question 1. What are monosaccharides?
Solution. Monosaccharides are the simplest units of carbohydrates. They are made up of hydrogen, carbon and oxygen atoms and are the building blocks of more complex carbohydrates. Monosaccharides containing an aldehyde group are known as aldoses and those containing a keto group are known as ketoses. Monosaccharides are further classified as trioses, tetroses, pentoses, hexoses, and heptoses according to the number of carbon atoms they contain. For example, a ketose containing 3 carbon atoms is called ketotriose and an aldose containing 3 carbon atoms is called aldotriose.
Monosaccharides are used to produce and store energy. Most organisms create energy by breaking down the monosaccharide glucose and harvesting the energy released from the bonds.
Examples of Monosaccharides are glucose, fructose, and galactose.
Question 2. What are reducing sugars?
Solution. The carbohydrates that can reduce Fehling's solution and Tollen's reagent are known as reducing sugars. All monosaccharides and disaccharides, excluding sucrose, are reducing sugars.
These carbohydrates reduce Fehling's solution to red precipitate of $\mathrm{Cu} 2 \mathrm{O}$ or Tollen's reagent to metallic $\mathrm{Ag}$.
Question 3. Write two main functions of carbohydrates in plants.
Solution. Two main functions of carbohydrates in plants are:
(i) Polysaccharides such as starch in plants serve as storage molecules.
(ii) Cellulose, a polysaccharide, is used in building the cell wall.
Question 4. Classify the following into monosaccharides and disaccharides.
Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Solution. Monosaccharides: Monosaccharides are the simplest units of carbohydrates which cannot be hydrolyzed into simpler compounds.
So from the given options Ribose, 2-deoxyribose, galactose, fructose are monosaccharides
Disaccharides: A disaccharide is a carbohydrate that is formed when two monosaccharides are joined together and a molecule of water is removed from the structure.
Therefore Maltose, lactose are disaccharides from the given options.
Question 5. What do you understand by the term glycosidic linkage?
Solution. Glycosidic linkage refers to the linkage formed between two monosaccharide units through an oxygen atom by the loss of a water molecule. It is the condensation of the hydroxyl group of two monosaccharides to form a link between them.
A sucrose molecule contains two monosaccharide units $\alpha$-glucose and $\beta$-fructose which are joined together by a glycosidic linkage.

Question 6. What is glycogen? How is it different from starch?
Solution. Glycogen is a carbohydrate (polysaccharide). In animals, carbohydrates are stored as glycogen. When body needs glucose, enzymes present in the body break glycogen down to glucose.
Starch is a carbohydrate consisting of two components - amylose (15 - $20 \%$ ) and amylopectin $(80-85 \%)$ whereas glycogen consists of only one component whose structure is similar to amylopectin.
Question 7. What are the hydrolysis products of:
(i) sucrose and
(ii) lactose
Solution. (i) Sucrose molecule on hydrolysis gives one molecule of $\alpha-\mathrm{D}$ glucose and one molecule of $\beta-$ D - fructose. Hydrolysis breaks the glycosidic bond converting sucrose into glucose and fructose.

(ii) Lactose on hydrolysis gives $\beta-\mathrm{D}-$ galactose and $\beta-\mathrm{D}-$ glucose.
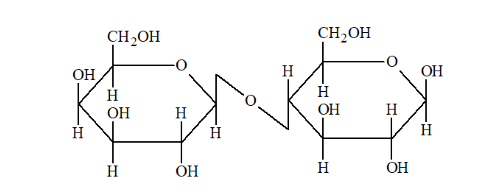
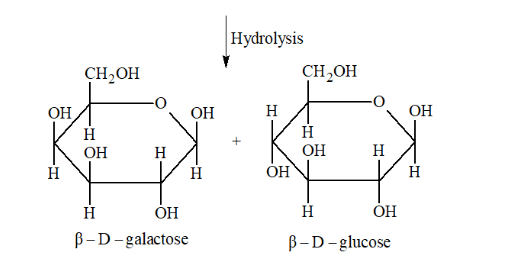
Question 8. What is the basic structural difference between starch and cellulose?
Solution. Starch consists of two components - amylose, and amylopectin.
Amylose is a long linear chain of $\alpha-D-(+)-$ glucose units joined by $C 1-C 4$ glycosidic linkage ( $\alpha-$ link).

Amylopectin is a branched-chain polymer of $\alpha$-D-glucose units, in which the chain is formed by $C 1-C 4$ glycosidic linkage and the branching occurs through $C 1-C 6$ glycosidic linkage.
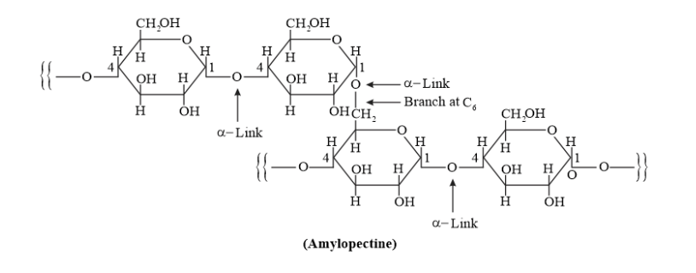
On the other hand, cellulose is a straight-chain polysaccharide of $\beta$-D-glucose units joined by C1 - C4 glycosidic linkage ( $\beta$-link).

Question 9. What happens when D-glucose is treated with the following reagents?
(i) HI
(ii) Bromine water
(iii) $\mathrm{HNO}_{3}$
Solution. (i) When D-glucose is heated with HI for a long time, n-hexane is formed. This shows that all the six-carbon atoms are linked in a straight chain.
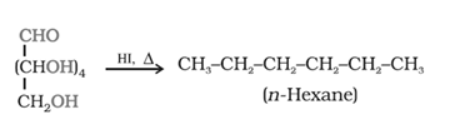
(ii) When D-glucose is treated with $\mathrm{Br}_{2}$ water (mild oxidising agent), D- gluconic acid is produced.
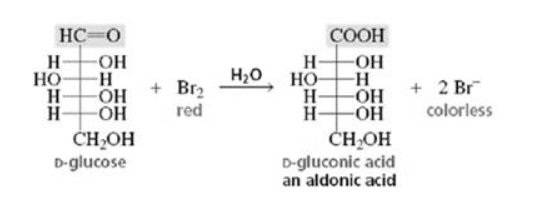
(iii) On being treated with $\mathrm{HNO}_{3}$, D-glucose get oxidised to give saccharic acid as product.
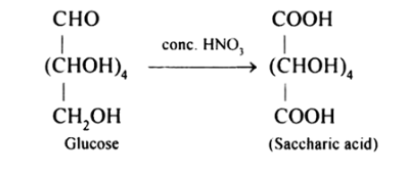
Question 10. Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Solution. (1) Aldehydes give 2,4 - DNP test, Schiff's test, and react with $\mathrm{NaHSO}_{4}$ to form the hydrogen sulphite addition product. However, glucose despite having aldehydes does not undergo these reactions.
(2) The pentaacetate of glucose does not react with hydroxylamine. This shows the absence of a free $-\mathrm{CHO}$ group from glucose.
(3) Glucose exists in two crystalline forms - $\alpha$ and $\beta .$ The $\alpha$-form (m.p.= $419 \mathrm{~K}$ ) crystallises from a concentrated solution of glucose at $303 \mathrm{~K}$ and the $\beta$-form (m.p. $=423 \mathrm{~K}$ ) crystallises from a hot and saturated aqueous solution at $371 \mathrm{~K}$. This behaviour cannot be explained by the open chain structure of glucose.
Question 11. What are essential and non-essential amino acids? Give two examples of each type.
Solution. Essential amino acids are required by the human body, but these cannot be synthesised in the body. They must be taken through food. For example: valine and leucine
Non-essential amino acids are also required by the human body, but they can be synthesized in the body. For example: glycine, and alanine
Question 12. Define the following as related to proteins
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Solution. (i) Peptide linkage:
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water $\left(\mathrm{H}_{2} 0\right)$. This is a condensation reaction and usually occurs between amino acids.
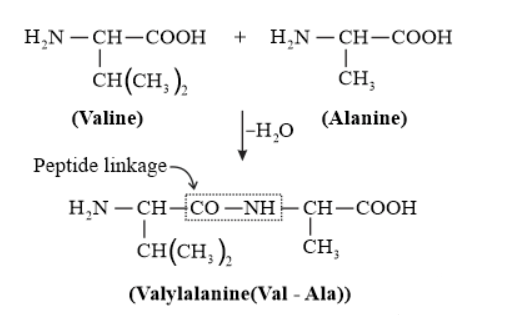
(ii) $\quad$ Primary structure:
The primary structure of a protein is the specific sequence in which various amino acids are present in it. It is the sequence of linkages between amino acids in a polypeptide chain. The sequence in which amino acids are arranged is different in each protein. A change in the sequence creates a different protein. Also the sequence of amino acids in a protein determines its function and biological activity.
(iii) Denaturation:
In a biological system, a protein is found to have a unique 3-dimensional structure and a unique biological activity. This is said to be native form of protein. When any protein present in its native form is subjected to a physical change (Change in temperature) or chemical changes like change in $\mathrm{pH}$, its hydrogen bonds are broken. This disturbance unfolds the globules and uncoils the helix of the protein molecule. This makes the protein lose its biological activity. This loss of biological activity by the protein is known as denaturation. During denaturation, the secondary and tertiary structures of the protein get destroyed, but the primary structure remains unaltered. Due to denaturation the globular proteins are converted into fibrous proteins. Hence denaturation leads to coagulation.
One of the examples of denaturation of proteins is the coagulation of egg white when an egg is boiled.
Question 13. What are the common types of secondary structure of proteins?
Solution. Secondary structure of proteins are of two types:
(i) $\alpha$-helix structure
(ii) $\beta$-pleated sheet structure
$\alpha-$ Helix structure:
In an $\alpha$ helix, the carbonyl $(\mathrm{C}=0)$ of one amino acid forms a hydrogen bond with the $-\mathrm{NH}$ group of an amino acid that is four down the chain. The $\mathrm{R}$ groups of the amino acids stick outward from the $\alpha$ helix, where they are free to interact
$\beta$ - pleated sheet structure:
This structure is called so because it looks like the pleated folds of drapery. In this structure, all the peptide chains are stretched out to nearly the maximum extension and then laid side by side. These peptide chains are held together by intermolecular hydrogen bonds.
In a $\beta$ pleated sheet, two or more segments of a polypeptide chain line up next to each other, forming a sheet-like structure held together by hydrogen bonds. The hydrogen bonds form between carbonyl and amino groups of backbone, while the R groups extend above and below the plane of the sheet.
Question. 14. What type of bonding helps in stabilising the $\alpha$-helix structure of proteins?
Solution: The intramolecular hydrogen bonds formed between the $-\mathrm{NH}$ group of each amino acid residue and the carbonyl $(\mathrm{C}=0)$ group of the adjacent turns of the $\alpha$-helix help in stabilising the helix.
Question 15. Differentiate between globular and fibrous proteins.
Solution.
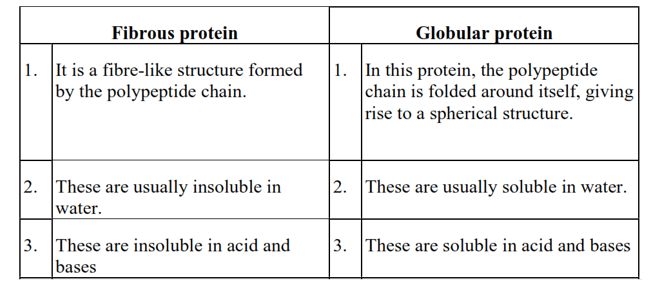

Question 16. How do you explain the amphoteric behaviour of amino acids?
Solution. In aqueous solution, the carboxyl group of an amino acid can lose a proton and the amino group can accept a proton to given a dipolar ion known as zwitter ion.

In zwitter ionic form the amino acid show amphoteric behaviour (can act both as an acid and as a base).
Example: In the zwitter ion given below, the $\mathrm{COO}^{-}$ion accepts a proton to form a cation in the presence of acidic medium. While in basic medium the $\mathrm{NH}_{3}^{+}$ion loses a proton to form anion.

Thus, amino acids show amphoteric behavior.
Question 17. What are enzymes?
Solution. Enzymes are proteins that catalyse biological reactions. They are highly specific in nature that catalyse only a specific reaction for a particular substrate. Enzymes can catalyze several million reactions per second. Enzymes are usually named after the particular substrate or class of substrate and sometimes after the particular reaction.
For example, the enzyme used to catalyse the hydrolysis of maltose into glucose is named as maltase.
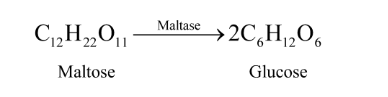
Question 18. What is the effect of denaturation on the structure of proteins?
Solution. In a biological system, a protein is found to have a unique 3 -dimensional structure and a unique biological activity. This is said to be native form of protein. Denaturation occurs when any protein present in its native form is subjected to a physical change (Change in temperature) or chemical changes like change in $\mathrm{pH}$ and its hydrogen bonds are broken. This disturbance unfolds the globules and uncoils the helix of the protein molecule. This makes the protein lose its biological activity. During denaturation, the secondary and tertiary structures of the protein get destroyed, but the primary structure remains unaltered. Due to denaturation the globular proteins are converted into fibrous proteins. Hence denaturation leads to coagulation.
Question 19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Solution. Vitamins are classified into two groups on the basis of their solubility in water or fat. They are:
(i) Fat-soluble vitamins:
Vitamins that are soluble in fat and oils, but not in water, belong to this group. Vitamins A, D, E, and Kare examples of fat-soluble vitamins.
(ii) Water-soluble vitamins:
Vitamins that are soluble in water belong to this group. B group vitamins $\left(\mathrm{B}_{1}, \mathrm{~B}_{2}, \mathrm{~B}_{6}, \mathrm{~B}_{12}\right.$, etc. $)$ and vitamin $\mathrm{C}$ are examples of fat-soluble vitamins.
Biotin (vitamin $\mathrm{H}$ ) is an exception that is neither soluble in water nor in fat.
Vitamin $\mathrm{K}$ is responsible for the coagulation of blood.
Question 20. Why are vitamin A and vitamin C essential to us? Give their important sources.
Solution. The deficiency of vitamin A leads to Xerophthalmia (hardening of the cornea of the eye) and night blindness. The deficiency of vitamin C causes Scurvy (bleeding of gums).
The sources of vitamin $A$ are fish, cod liver oil, butter, milk, carrots.
The sources of vitamin $C$ are citrus fruits, amla, lemon and green leafy vegetables.
Question 21. What are nucleic acids? Mention their two important functions.
Solution. Nucleic acids are biomolecules found in the nuclei of all living cells, as one of the
constituents of chromosomes. Nucleic acids are of two types:
(i) Deoxyribonucleic acid (DNA)
(ii) Ribonucleic acid (RNA)
Nucleic acids are also known as polynucleotides as they are long-chain polymers of nucleotides.
Two important functions of nucleic acids are:
(i) DNA is responsible for heredity. It is the process of transmission of inherent characters from one generation to the next.
(ii) Nucleic acids (both DNA and RNA) are responsible for synthesis of proteins in a cell. These are needed for the growth and maintenance of our body. Even though the proteins are actually synthesized by the various RNA molecules in a cell, the message for the synthesis of a particular protein is given by a DNA molecule.
Question 22. What is the difference between a nucleoside and a nucleoside?
Solution: A nucleoside is formed by the attachment of base to $1^{\prime}$ position of sugar.
Nucleoside $=$ Sugar $+$ Base
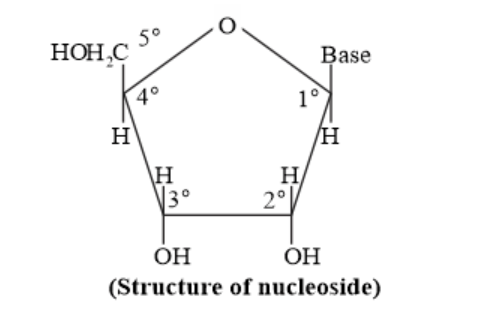
On the other hand, all the three basic components of nucleic acids (i.e., pentose sugar, phosphoric acid, and base) are present in a nucleotide. Nucleotides are obtained by esterification of $\mathrm{C} 5^{\prime}-\mathrm{OH}$ group of the pentose sugar by phosphoric acid.
Nucleotide $=$ Sugar $+$ Base $+$ Phosphoric acid
Question 23. The two strands in DNA are not identical but are complementary. Explain.
Solution. In DNA helical structure, the two strands are held together by hydrogen bonds between specific pairs of bases. Cytosine forms a hydrogen bond with guanine, while thymine forms a hydrogen bond with adenine. Thus as a result, the two strands are complementary to each other. Also the sequence of bases in one strand automatically determines that of the other
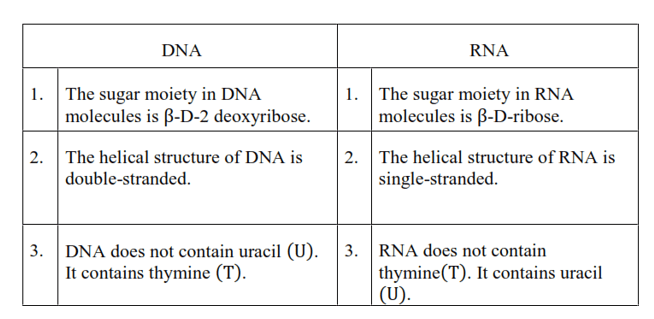
Question 24. Write the important structural and functional differences between DNA and RNA.
Solution. The structural differences between DNA and RNA are as follows:
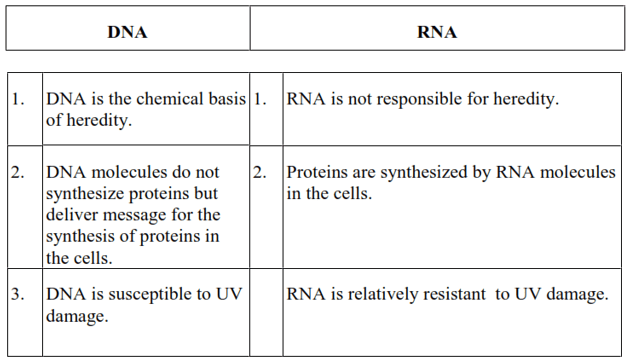
The functional differences between DNA and RNA are as follows
Question 25. What are the different types of RNA found in the cell?
Solution. The different types of RNA found in the cell are:
(i) Messenger RNA (m-RNA):
Messenger RNA carries the genetic message code from DNA to ribosomes. It is produced by the DNA; m-RNA is also single stranded and constitutes about $15 \%$ of total RNA.
(ii) Ribosomal RNA (r-RNA):
Ribosomal RNA is found in the ribosomes. It is usually associated with protein to form the ribosomes. It is synthesised in the nucleus by DNA. It is single stranded, comprising about $80 \%$ of total RNA. It is metabolically stable.
(iii) Transfer RNA (t-RNA):
Transfer RNA is synthesised in nucleus by DNA. It is soluble RNA and is single stranded. It constitutes about $5 \%$ of total RNA and has a very short life.
Also Read,
Class 12 Chemistry Notes Free PDF.
Class 12 Chemistry Book Chapterwise Free PDF.
Class 12 Chemistry Exemplar Chapterwise Free PDF.
If you have any Confusion related to NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules PDF then feel free to ask in the comments section down below.
To watch Free Learning Videos on Class 12 Chemistry by Kota’s top IITan’s Faculties Install the eSaral App
