
NCERT Solutions for Class 11 Biology chapter 9 Biomolecules PDF
Hey, are you a class 11 student and looking for ways to download NCERT Solutions for Class 11 Biology chapter 9 Biomolecules PDF? If yes. Then read this post till the end.In this article, we have listed NCERT Solutions for Class 11 Biology chapter 9 Biomolecules in PDF that are prepared by Kota’s top Doctor’s Faculties by keeping Simplicity in mind.
If you want to learn and understand class 11 Biology chapter 9 "Biomolecules" in an easy way then you can use these solutions PDF.
NCERT Solutions helps students to Practice important concepts of subjects easily. Class 11 Biology solutions provide detailed explanations of all the NCERT questions that students can use to clear their doubts instantly.
If you want to score high in your class 11 Biology Exam then it is very important for you to have a good knowledge of all the important topics, so to learn and practice those topics you can use eSaral NCERT Solutions.
In this article, we have listed NCERT Solutions for Class 11 Biology chapter 9 Biomolecules PDF that you can download to start your preparations anytime.
So, without wasting more time Let’s start.
Download NCERT Solutions for Class 11 Biology chapter 9 Biomolecules PDF
Question 1. What are macromolecules? Give examples.
Solution: -Macromolecules are the molecules which have the molecular weight in the range of 1000 daltons (Da) or above 1000 Dalton and are formed by the polymerization of several similar or different types of molecules.
- They are found in the acid-insoluble fraction of the tissue extract.
-Examples of the macromolecules are:
1. Proteins
2. Lipids
3. Nucleic acid
4. Polysaccharide
Question 2. Illustrate a glycosidic, peptide and phosphodiester bond.
Solution:


Question 3. What is meant by the tertiary structure of proteins?
Solution. The tertiary structure is a structure formed in proteins when the beta-pleated sheets or alpha helix (secondary structures of proteins) folds further to form a hollow woolen balllike structure. (1marks for correct explanation 1 marks for illustration)
Diagram showing the folding of proteins in the tertiary structure.

Question 4. Find and write down structures of 10 interesting small molecular weight biomolecules. Find if there is any industry which manufactures the compounds by isolation. Find out who are the buyers.
Solution.(a)



Question 5. Proteins have primary structure. If you are given a method to know which amino acid is at either of the two termini (ends) of a protein, can you connect this information to purity or homogeneity of a protein.
Solution. The primary structure of protein constitutes amino acids. The two terminal of proteins are amino terminal and carboxyl terminal. If we have the information about the amino acids present at the two ends of the protein the upon comparing the given information about the terminal amino acid. It can be said that the protein which is isolated is pure or homogeneous if it has the same amino acids at the amino terminal and carboxyl terminal.
Question 6. Find out and make a list of proteins used as therapeutic agents. Find other applications of proteins (e.g. cosmetics etc.).
Solution. List of proteins used as therapeutic agents:
1. Insulin
2.Oxytocin
3.Antidiuretic Hormone (ADH),
4. Thrombin
5.Fibrinogen
6.Renin
7.Immunoglobulin
8. Diastase
9.Streptokinase
Other applications:
i. Cosmetics:- Proteins such as collagen and elastin are used in anti-ageing creams. Keratin and cystine protein is present in shampoos, which prevents the hair from damage.
ii. Sweeteners:-Certain proteins are artificial sweeteners, and taste modifiers. There are seven known proteins which are artificial sweeteners. Examples: Razzein ,Thaumatin, Monelin, Curculin, Mabinlin, Miraculin and Pentadin.
iii. Dietary proteins:- Protein is essential for body-building. Protein isolates such as whey, pea, soy, casein etc. are consumed before or after extensive exercise as they help in the formation of muscle.
Question 7. Explain the composition of triglyceride.
Solution. When three fatty acids are esterified with glycerol, it is known as triglycerides.
Glycerol is the compound which has three carbon atoms having three alcohol attached to. them. Whereas fatty acids are the high carbon containing acids.
- When the alcohol groups of glycerol and carboxylic groups of fatty acids react to form an ester bond with the elimination of water molecule which is formed is triglycerides.
Diagram showing composition and bonding in triglycerides
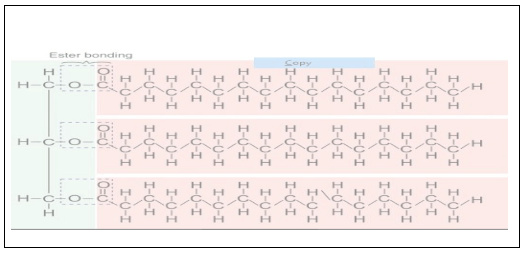
Question 8. Can you describe what happens when milk is converted into curd or yoghurt, from your understanding of proteins?
Solution: -When the inoculum of Lactobacillus bacteria is added to lukewarm milk, the formation of curd takes place. The Lactobacillus multiplies and secretes lactic acid. The lactic acid decreases the $\mathrm{pH}$ of the milk and makes it acidic.
-Due to this decrease in $\mathrm{pH}$, the secondary and tertiary structure of milk protein "casein" gets denatured and gets precipitated.
Question 9. Can you attempt building models of biomolecules using commercially available atomic models (ball and stick models)?
Solution. -Structure of biomolecules can be represented using ball and stick model.
-The bonds which hold the atoms are represented by sticks, whereas the atoms are depicted as balls. In order to show different molecules, different colour of balls can be used.
Question 10. Attempt titrating an amino acid against a weak base and discover the number of dissociating (ionizable) functional groups in the amino acid.
Solution. -When the weak base is added to the amino acid solution, the carboxylic group of amino acid dissociates and releases the hydrogen ion in order to balance the $\mathrm{pH}$ of the medium.
-When the $\mathrm{pH}$ of the medium reduces and becomes acidic, the amino group takes up the hydrogen ion from the medium and balances the $\mathrm{pH}$.
- When all the amino groups are positively charged, and all the carboxylic groups are aegatively charged in an amino acid, then that state of amino acid is referred to as “Zwitter-ion."
$\underline{\text { Diagram showing titration of amino acid with a weak base }}$

Question 11. Draw the structure of the amino acid alanine.
Solution. Diagram showing structure of Alanine amino acid

Question 12. What are gums made of? Is fevicol different?
Solution. Gums are made up of polymeric substances which are secreted from the plants. It is a secondary metabolite which is formed as a result of a metabolic pathway of the plants. Fevicol is an adhesive which has a similar function as that of gum the difference between the two is that gum is a plant-based product, and fevicol is a synthetic product.
Question 13. Find out a qualitative test for proteins, fats and oils, amino acids and test any fruit juice, saliva, sweat and urine for them.
Solution: Qualitative tests for detecting the proteins, amino acids and fats are as follows:
Biuret test for proteins: The biuret test is the chemical test performed in order to detect the presence of protein in the sample.
-Biuret reagent reacts with peptide bond of proteins and forms a purple coloured complex.
ii. Grease test for Fats: When the drop of oil or fat is applied on a brown paper, it makes the paper translucent. This confirms the presence of fats or oil in the sample.
iii. Ninhydrin test for amino acids: Ninhydrin reagent (2,2-dihydroxyindane-1,3-dione) reacts with the primary and secondary amino group of amino acids.
It forms a dark blue coloured dye which is known as Ruheman's purple, confirming the presence of amino acid in the sample.
Exception: Proline amino acid forms a yellow complex with ninhydrin due to the presence of the guanidine ring.


Question 14. Find out how much cellulose is made by all the plants in the biosphere and compare it with how much of paper is manufactured by man, and hence, what is the consumption of plant material by man annually. What a loss of vegetation?
Solution. -Cellulose is the structural component of the plants. The main cover of the biosphere is made up of plants. There are approximately $3.04$ trillion trees present on the biosphere and the amount of cellulose that is produced by them is 100 billion tonnes.
- It requires $16-20$ trees to make a ton of paper. Total 180 million tonnes of cellulose is produced every year, which involves the sacrifice of approximate 5 crore trees. Besides, that there is a dependency on plants for the plant products such as timber, food, medicines etc., which is also contributing to the loss of vegetation.
Question 15. Describe the important properties of enzymes.
Solution. Properties of enzymes are described as follows:
1. Chemical nature:
- Enzymes are the nitrogenous compounds that are made up of protein. All enzymes are protein in nature, but not all proteins are enzymes.
-Enzymes work as biocatalyst which means they help in accelerating the biochemical reaction by forming the enzyme-substrate complex and decreasing the activation energy of the reaction.
2. Molecular welght:
-Enzymes are categorized as macromolecules as they are proteins.
-The molecular weight of the enzyme ranges from 1000 dalton to 1,00,000 Dalton or above.
3.Changeless form:
-Enzymes are not consumed in the chemical reaction.
-They combine with the substrate forms enzyme-substrate complex and ronverts into the product.
4. Substrate specificity:
- Different biochemical reactions are catalyzed by a different enzyme. Every enzyme has its own substrate like every lock has its own key. The theory related to the enzyme-substrate complex is referred to as a lock and key hypothesis. $\quad$
5. Heat sensitivity:
-Enzymes are sensitive to temperature. They work best at the optimum temperature that is from $30-40$ degree Celsius. Enzymes get inhibited at low temperature, and they get denatured at high temperature.
6. pH sensitivity:
Enzymes are sensitive to $\mathrm{pH}$ they activity of enzymes is best at the optimum temperature they undergo denaturation when they are subjected to high $\mathrm{pH}$ or low $\mathrm{pH}$.
Also Read,
Download Class 11 Chemistry Notes pdf.
Download Class 11 Biology Book Chapterwise pdf.
Download Class 11 Biology Exemplar Chapterwise pdf.
If you have any Confusion related to NCERT Solutions for Class 11 Biology chapter 9 Biomolecules PDF then feel free to ask in the comments section down below.
To watch Free Learning Videos on Class 11 Biology by Kota’s top Doctor’s Faculties Install the eSaral App
