JEE Advanced Previous Year Questions of Chemistry with Solutions are available at eSaral. Practicing JEE Advanced Previous Year Papers Questions of Chemistry will help the JEE aspirants in realizing the question pattern as well as help in analyzing weak & strong areas.
Simulator
Previous Years JEE Advance Questions
Q. The correct acidity order of the following is :
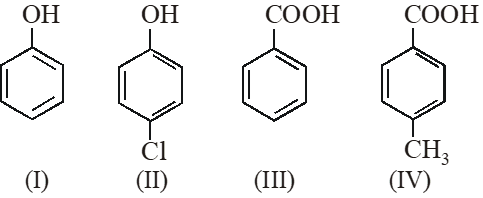 (A) (III) > (IV) > (II) > (I)
(B) (IV) > (III) > (I) > (II)
(C) (III) > (II) > (I) > (IV)
(D) (II) > (III) > (IV) > (I)
[IIT-JEE-2009]
(A) (III) > (IV) > (II) > (I)
(B) (IV) > (III) > (I) > (II)
(C) (III) > (II) > (I) > (IV)
(D) (II) > (III) > (IV) > (I)
[IIT-JEE-2009]
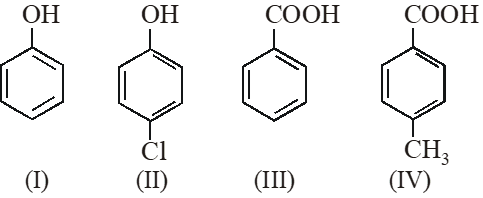 (A) (III) > (IV) > (II) > (I)
(B) (IV) > (III) > (I) > (II)
(C) (III) > (II) > (I) > (IV)
(D) (II) > (III) > (IV) > (I)
[IIT-JEE-2009]
(A) (III) > (IV) > (II) > (I)
(B) (IV) > (III) > (I) > (II)
(C) (III) > (II) > (I) > (IV)
(D) (II) > (III) > (IV) > (I)
[IIT-JEE-2009]
Ans. (A)
(A) Acidity $\propto-R /-I \propto \frac{1}{+R /+I}$
Q. The correct stability order of the following resonance structures is
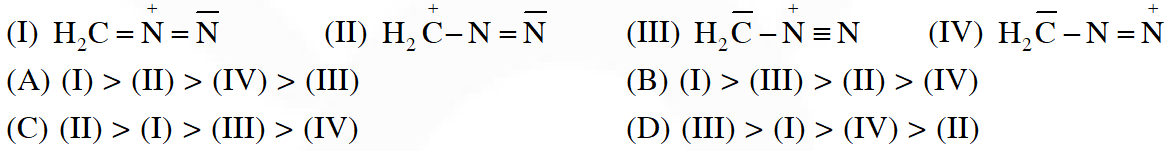 [IIT-2009]
[IIT-2009]
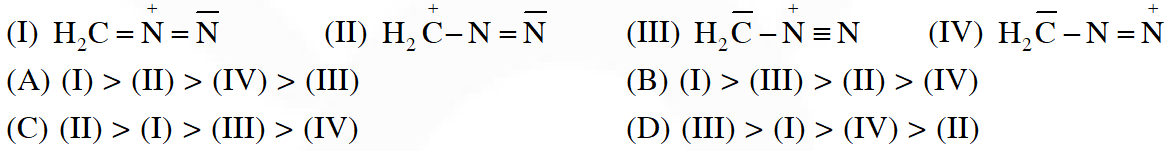 [IIT-2009]
[IIT-2009]
Ans. (B)
Q. Amongst the following, the total number of compounds soluble in aquesous NaOH is:
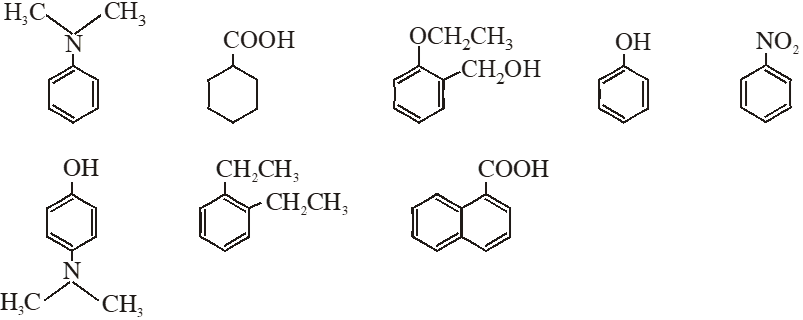 [IIT-JEE-2010]
[IIT-JEE-2010]
 [IIT-JEE-2010]
[IIT-JEE-2010]
Ans. 4
(4) Compound which is more acidic than water is soluble in NaOH
Q. The total number of contributing structures showing hyperconjugation (involving C–H bonds) for the following carbocation is.
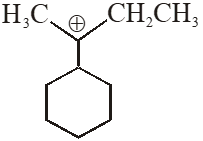 [IIT-2011]
[IIT-2011]
 [IIT-2011]
[IIT-2011]
Ans. 6
$6 \propto \mathrm{H}$ ore present.
Q. Among the following compounds, the most acidic is –
(A) p-nitrophenol
(B) p-hydroxybenzoic acid
(C) o-hydroxybenzoic acid
(D) p-toluic acid
[IIT-JEE-2011]
Ans. (C)
Q. Which of the following molecules, in pure from , is (are) unstable at room temperature ?
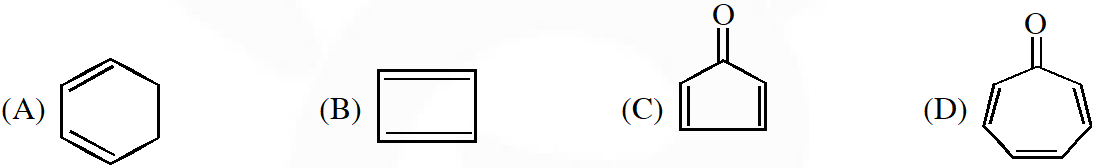 [IIT-2012]
[IIT-2012]
 [IIT-2012]
[IIT-2012]
Ans. (B,C)
Anti - Aromatic : B,C
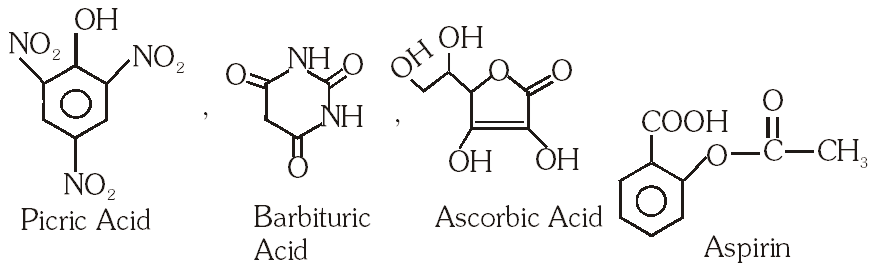
Q. The carboxyl functional group (–COOH) is present in –
(A) picric acid
(B) barbituric acid
(C) ascorbic acid
(D) aspirin
[IIT-JEE-2012]
Ans. (D)

Q. Identify the binary mixtures (s) that can be separated into the individual compounds, by differential extraction, as shown in the given scheme -
 (A) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$
(B) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$
(C) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$
(D) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{COOH}$
[IIT-JEE-2012]
(A) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$
(B) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$
(C) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$
(D) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{COOH}$
[IIT-JEE-2012]
 (A) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$
(B) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$
(C) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$
(D) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{COOH}$
[IIT-JEE-2012]
(A) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$
(B) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$
(C) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$
(D) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{OH}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{COOH}$
[IIT-JEE-2012]
Ans. (B,D)
Q. The hyperconjugative stbilities of tert-butyl cation and 2-butene, respectively, are due to –
(A) $\sigma \rightarrow \mathrm{p}$ (empty) and $\sigma \rightarrow \pi$ electron delocalisations
(B) $\sigma \rightarrow \sigma$ and $\sigma \rightarrow \pi$ electron delocalisations
(C) $\sigma \rightarrow \mathrm{p}(\text { filled })$ and $\sigma \rightarrow \pi$ electron delocalisations
(D) $\mathrm{p}$ (filled) $\rightarrow \sigma$ and $\sigma \rightarrow \pi$ electron delocalisations
[IIT-2013]
Ans. (A)
Q. The total number of lone-pairs of electrons in melamine is -
[IIT-2013]
Ans. 6


Q. The compound that does NOT liberate $\mathrm{CO}_{2}$, on treatment with aqueous sodium bicarbonate solution, is –
(A) Benzoic acid
(B) Benzenesulphonic acid
(C) Salicylic acid
(D) Carbolic acid (phenol)
[JEE-ADVANCED-2013]
Ans. (D)
(D) Compound which is more acidic than $\mathrm{H}_{2} \mathrm{CO}_{3}$ liberate $\mathrm{CO}_{2}$ gas.
Q. Hydrogen bonding plays a central role in the following phenomena
(A) Ice floats in water
(B) Higher Lewis basicity of primary amines than tertiary amines in aqueous solutions
(C) Formic acid is more acidic than acetic acid
(D) Dimerisation of acetic acid in benzene
[JEE-ADVANCED-2014]
Ans. (A,B,D)
Q. The number of resonance structures for N is :
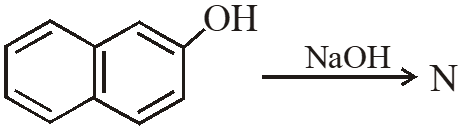 [IIT-2015]
[IIT-2015]
 [IIT-2015]
[IIT-2015]
Ans. 9

Comments
pharmacy online
Oct. 24, 2023, 6:35 a.m.
I am really enjoying the theme/design of your website. Do you ever run into any web browser compatibility issues? A small number of my blog readers have complained about my blog not working correctly in Explorer but looks great in Safari. Do you have any solutions to help fix this issue?
VincentMum
Feb. 8, 2023, 1:32 p.m.
Thanks a lot, Quite a lot of write ups.
https://ouressays.com/ mba dissertation writing
Error
Sept. 19, 2022, 3 p.m.
solution k liye app kyu download kree koi mtlb Banta hai free krooo bahot revolution la rhe ho toh
Yuvraj prajapati
July 21, 2020, 7:12 p.m.
Nice questions and cake samay me NEET k liye hi achah bn payega
