
Get Aldehydes, Ketones and carboxylic acids class 12 important questions and answers for Boards exams. View the Important Question bank for Class 11 & 12 Chemistry. These important questions will play significant role in clearing concepts of Chemistry syllabus. This question bank is designed by keeping, NCERT in mind and the questions are updated with respect to upcoming Board exams. You will get here all the important questions for class 12 chemistry chapters along with the solved important questions of Aldehydes, Ketoness and Carboxylic Acids.
Click Here for Detailed Chapter-wise Notes of Chemistry for Class 12th, JEE & NEET.
You can access free study material for all three subjects: Physics, Chemistry and Mathematics.
Click Here for Detailed Notes of any chapter.
eSaral provides you complete edge to prepare for Board and Competitive Exams like JEE, NEET, BITSAT, etc.
We have transformed classroom in such a way that a student can study anytime anywhere. With the help of AI we have made the learning Personalized, adaptive and accessible for each and every one.
Visit eSaral Website to download or view free study material for JEE & NEET.
Also get to know about the strategies to Crack Exam in limited time period.
Q. Give the IUPAC names of:
Ans. (a) Diacetone alcohol, (b) Crotonaldehy de.
(a) $\quad 4$ - Hy droxy - 4 -methylpentan-2-one
(b) $\quad$ But- 2 -en- 1 -al.
Q. Name one reagent used to distinguish acetaldehyde from acetone.
Ans. Tollen’s reagent or Fehling’s solution.
Q. Name one reagent used to convert toluene into benzaldehyde.
Ans. Chromyl chloride in $\mathrm{CS}_{2}\left(\mathrm{CrO}_{2} \mathrm{Cl}_{2} / \mathrm{CS}_{2}\right)$ or chromic trioxide in acetic anhydride $\left[\mathrm{CrO}_{3} /\left(\mathrm{CH}_{3} \mathrm{CO}\right)_{2} \mathrm{O}\right]$ followed by acid or alkaline hydrolysis.
Q. What type of aldehydes and ketones undergo aldol condensation?
Ans. Aldehydes and ketones containing $\alpha$ -hydrogens.
Q. Which type of aldehydes undergo Cannizzaro reaction?
Ans. Aromatic and aliphatic aldehydes which do not contain $\alpha$ hydrogens
Q. Which alkene on reductive ozonolysis gives acetone as the only product?
Ans.


Q. Write the IUPAC name of


Ans. 2-Methylpentan-3-one
Q. To what oxidation state does ethanal reduce $C u(\mathrm{II}) ?$
Ans. +1 oxidation state
Q. Write the structure and give the IUPAC names of the following compounds. (a) Cinnamic acid (b) Crotonic acid (c) Oxalic acid.
Ans.


Q. Why does benzoic acid not undergo Friedel-Crafts reaction ?
Ans. (a) Due to deactivation of the benzene ring by electronwithdrawing effect of the $-C O O H$ group.
$(b)$ $A I C l_{3}$ gets bonded to the $-C O O H$ group.
Q. Why carboxylic acid do not form oximes ?
Ans. Due to resonance between lone pairs of electrons on the -atom of the group and the carboxyl carbon is less electrophilic than carbonyl carbon in aldedhydes and ketones. Therefore, nucleophilic addition of to the group of carboxylic acids does not occur and hence carboxylic acids do not form oximes.
Q. Give a suitable example of Hell-Volhard Zelinsky reaction.
Ans.


Q. Carbonyl compounds are more polar than alcohols although electronegativity difference between C and O atoms is less than O and H atoms. Explain.
Ans. In the carbonyl group $(>C=O),$ the $\pi$ -electron pair is loosely held and can be readily shifted to the oxygen atom. It is not the case with the alcoholic $(O-H)$ group. Therefore, carbonyl compounds are more polar and have higher dipole moment values $(2.3-2.8 D)$ than the alcohols $(1.6-1.8 D)$
Q. Give the IUPAC name of (a) Isobutyraldehy de (b) Acrolein (c) Valeraldehyde.
Ans.


Q. Which alkene upon reductive ozonolysis will give acetone as the product ?
Ans. 2, 3-dimethylbut-2-ene will form acetone by reductive ozonolysis


Q. Formaldehyde gives cannizzaro’s reaction while acetaldehyde does not. Why ?
Ans. Formaldehyde does not have an $\alpha$ -hydrogen. It therefore, takes part in cannizzaro's reaction when heated with strong $N a O H$ to form a methyl alcohol and sodium formate mixture. Acetaldehyde fails to react since it has $\alpha$ -hydrogens present.
Q. What happens when ethylbenzene is heated with acidified $K_{2} C r_{2} O_{7} ?$
Ans. Benzoic acid is formed.


Q. Why is benzoic acid a stronger acid than acetic acid?
Ans. The $K_{a}$ value of benzoic acid $\left(6.3 \times 10^{-5}\right)$ is more than that of
acetic acid $\left(1.75 \times 10^{-5}\right) .$ Actually, $C_{6} H_{5}$ group with $-I$
effect in facilitates the release of $H^{+}$ from benzoic acid while
$\mathrm{CH}_{3}$ group with $+I$ effect tends to retard it.
Q. Which out of acetic and peracetic acid is a stronger acid and why ?
Ans. Acetic acid $\left(p K_{a}=4.8\right)$ is stronger than peracetic acid
$\left(p K_{a}=8.2\right) .$ It is because after losing $H^{+}$ ion acetic acid molecule gets stabilised by resonance while the peracetate ion does not.


Q. Carboxylic acids do not give the characteristic reactions of carbonyl group.
Ans. Due to the presence of lone pairs of electrons on the oxygen atom of the OH group, the carboxylic acids are stabilized by resonance.
 As a result, the double bond character of the bond in carboxylic acids is greatly reduced as compared to that in aldehydes and ketones. In other words, the carbonyl group in carboxylic acids is not a true carbonyl group as in aldehydes and ketones and hence does not give some of the characteristic reactions of the carbonyl group. For example, unlike aldehydes and ketones, carboxylic acids do not form oximes, hydrazones, semicarbazones etc.
As a result, the double bond character of the bond in carboxylic acids is greatly reduced as compared to that in aldehydes and ketones. In other words, the carbonyl group in carboxylic acids is not a true carbonyl group as in aldehydes and ketones and hence does not give some of the characteristic reactions of the carbonyl group. For example, unlike aldehydes and ketones, carboxylic acids do not form oximes, hydrazones, semicarbazones etc.
 As a result, the double bond character of the bond in carboxylic acids is greatly reduced as compared to that in aldehydes and ketones. In other words, the carbonyl group in carboxylic acids is not a true carbonyl group as in aldehydes and ketones and hence does not give some of the characteristic reactions of the carbonyl group. For example, unlike aldehydes and ketones, carboxylic acids do not form oximes, hydrazones, semicarbazones etc.
As a result, the double bond character of the bond in carboxylic acids is greatly reduced as compared to that in aldehydes and ketones. In other words, the carbonyl group in carboxylic acids is not a true carbonyl group as in aldehydes and ketones and hence does not give some of the characteristic reactions of the carbonyl group. For example, unlike aldehydes and ketones, carboxylic acids do not form oximes, hydrazones, semicarbazones etc.
Q. Write reactions and conditions for following conversions :
(a) Phenol to salicylic acid
(b) $\quad 2$ -propanone into 2 -methyl- 2 -propanol.
Ans. (a) By Kolbe's reaction. Sodium salt of phenol reacts with
$\mathrm{CO}_{2}$ under high pressure of $4-7$ atm, at about $400 \mathrm{K}$ to form salicylic acid.




Q. Write chemical tests to distinguish between formic acid and acetic acid.
Ans.




Q. Although both $>\mathrm{C}=\mathrm{C}<$ and $>\mathrm{C}=\mathrm{O}$ have a double bond, they exhibit different type of addition reactions. Explain.
Ans. The $>\mathrm{C}=\mathrm{C}<$ undergoes electrophilic addition reactions while $>\mathrm{C}=\mathrm{O}$ shows nucleophilic addition reactions. This difference in behaviour is due to the different shapes of their $\pi$ -electron clouds. The electron cloud of bond due to similar electronegativities of the two carbon atoms is almost symmetrical and surrounds both the carbon atoms equally. Consequently, if a reagent is to attack one of the carbon atoms of the bond, it has to pass through the electron cloud. since the -electron cloud consists of loosely held electrons, therefore, it can readily allow electrophiles to react. As a result, the typical reactions of are electrophilc addition reactions. In contrast, the electron cloud of the $\succ \mathrm{C}=0$ is unsymmetrical i.e., shifts towards oxygen due to greater electronegativity of O than C. As a result, the C-atom of the bond acquires a partial charge and hence is readily attacked by nucleophiles. Thus, the typical reactions of are nucleophilic addition reactions.
Q. What is the function of Roschelle salt in Fehling’s solution?
Ans. In alkaline medium, $C u^{2+}$ ions get precipitated as $C u(O H)_{2}$ To keep ions in solution in alkaline medium, Rochelle salt is added. The insoluble first formed goes into solution due to the formation of a soluble complex between ions and tartrate ion as shown below:


Q. Suggest a suitable oxidising agent for the conversion $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{C}=\mathrm{CHCOCH}_{3} \rightarrow\left(\mathrm{CH}_{3}\right)_{2} \mathrm{C}=\mathrm{CHCO}_{2} \mathrm{H}$
Ans. Alkaline $K M n O_{4},$ acidified $K_{2} C r_{2} O_{7}$ or $H N O_{3}$ cannot be used since all of these will cleave the molecule at the site of double bond giving a mixture of ketone/acids. The most suitable reagent for this oxidation is $\mathrm{NaOI} \quad\left(I_{2} / N a O H\right)$ since methyl ketones on treatment with NaOI undergo iodoform reaction to give iodoform along with the salt of a carboxylic acid having one carbon atom less than the starting methyl ketone.


Q. Fluorine is more electronegative than chlorine even than $p-$ fluorobenzoic acid a weaker acid than $p$ -chlorobenzoic acid. Explain
Ans. since halogens are more electronegative than carbon and also possess lone pairs of electrons, therefore, they exert both $-I$ and $+R$ -effects. Now in $F,$ the lone pairs of electrons are present in $2 p$ -orbitals but in $C l,$ they are present in $3 p-$ orbitals.
since $2 p$ -orbitals of $F$ and $C$ are of almost equal size, therefore, the $+R$ -effect is more pronounced in $p-$ fluorobenzoic acid than in -chlorobenzoic acid
 Thus, in $p$ -fluorobenzoic acid, $+R$ -effect outweighs the $-I-$ effect but is p-chlorobenzoic acid, it is the I-effect which outweighs the $+$ R-effect. Consequently acid is a weaker acid than p-chlorobenzoic acid.
Thus, in $p$ -fluorobenzoic acid, $+R$ -effect outweighs the $-I-$ effect but is p-chlorobenzoic acid, it is the I-effect which outweighs the $+$ R-effect. Consequently acid is a weaker acid than p-chlorobenzoic acid.
 Thus, in $p$ -fluorobenzoic acid, $+R$ -effect outweighs the $-I-$ effect but is p-chlorobenzoic acid, it is the I-effect which outweighs the $+$ R-effect. Consequently acid is a weaker acid than p-chlorobenzoic acid.
Thus, in $p$ -fluorobenzoic acid, $+R$ -effect outweighs the $-I-$ effect but is p-chlorobenzoic acid, it is the I-effect which outweighs the $+$ R-effect. Consequently acid is a weaker acid than p-chlorobenzoic acid.
Q. Give an example of a reaction where a Grignard reagent acts as a reducing agent.
Ans. If the Grignard reagent or the ketone contains branching at the $\alpha$ -carbon, sometimes the usual addition reaction does not occur due to steric hindrance. Instead reduction occur in which a hydride ion is transferred from the Grignard reagent to the ketone via a six-membered cyclic transition state in a manner similar to
M.P.V. reduction. For example, when isopropylmagnesium bromide is added to di-isopropyl ketone, the expected $3^{\circ}$ alcohol (i.e., tri-isopropylcarbinol) is not formed; instead the secondary alcohol, di-isopropylcarbinol is obtained by reduction of the keto group.


Q. Boiling points of carbonyl compounds lie between the parent alkanes and corresponding alcohols. Justify.
Ans. Alkanes are non-polar in nature and the attractive forces are weak vander Waal’s forces. In alcohols, intermolecular hydrogen bonding is present in the molecules. In the carbonyl compounds, only dipolar forces exist in the molecules. It is, therefore, quite obvious that the attractive forces in carbonyl compounds are more than in alkanes and less in comparison to alcohols. This justifies the trend in boiling point values in the members of these families.
Q. Halogen acids readily combine with alkenes to form addition products but fail to react with carbonyl compounds. Discuss.
Ans. Halogen acids (HX) react with alkenes to form haloalkanes but they fail to react with the carbonyl compounds. In fact, the attack does take place but the product is unstable and decomposes to form carbonyl compound along with halogen acid. Thus, the reaction is reversible.


Q. Out of benzaldehyde and propionaldehye which is more reactive towards nucleophilic addition ?
Ans. Propionaldehyde is more reactive than benzaldehyde towards nucleophilic addition. Actually, in benzaldehyde the aldehydic group has (mesomeric) effect. As a result, the electron density on the carbonyl carbon atom tends to increase and the nucleophile attack is more difficult as compared to propanal where alkyl group is attached to the aldehydic group.
Q. Chloral hydrate is a gem-diol but still stable. How will you account for it?
Ans. In chloral hydrate, the presence of three $C l$ atoms with $-I$ effect increases the magnitude of the positive charge on the carbonyl carbon atom. As a result, even a weak nucleophile like $H_{2} O$ attacks to form a hydrate. Moreover, there is weak intermolecular hydrogen bonding in the chloral hydrate which tends to increase its stability.


Q. Why does not formaldehyde take part in aldol condensation?
Ans. Aldol condensation involves the nucleophile attack of carbanion generated by one molecule of a particular carbonyl compound on the other molecule. For this, the carbonyl compound must have atleast one $\alpha$ -hydrogen present. since formaldehyde $(H C H O)$ has no such hydrogen present, it fails to take part in aldol condensation alongwith another carbonyl compound with atleast one -hydrogen atom e.g., formaldehyde and acetaldehyde.
Q. Why is it necessary to control the pH during the reaction of aldehydes and ketones with ammonia derivatives?
Ans. The addition of ammonia derivative $\left(N H_{2}-G\right)$ to aldehydes and ketones is done in weakly acidic medium (pH about 3.5). In case the medium is strongly acidic (pH close to 1 ), then the ammonia derivative will be also protonated and will not be able to act as a nucleophile.
Q. Benzophenone does not react with $\mathrm{NaHSO}_{3} .$ Explain.
Ans. The addition of $N a H S O_{3}$ on benzophenone $\left[\left(C_{6} H_{5}\right)_{2} C=O\right]$ involves the nucleophile attack of bisulphite $\left(H S O_{3}^{-}\right)$ ion. The phenyl groups act as hindrance to the attacking nucleophile. Therefore, benzophenone does not react with $\mathrm{NaHSO}_{3}$.
Q. Arrange the following in decreasing ease of hydration:
(a) $\mathrm{CH}_{3} \mathrm{CHO},\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CO}, \mathrm{HCHO}, \mathrm{CH}_{2} \mathrm{ClCOCH}_{3}, \mathrm{CH}_{2} \mathrm{ClCHO}$


Ans. Aldehydes are more easily hydrated than ketones. Further, the presence of electron withdrawing groups increases the ease of hydration of a carbonyl compound. In the light of this, the correct order is :
(a) $\mathrm{HCHO}>\mathrm{CH}_{2} \mathrm{ClCHO}>\mathrm{CH}_{3} \mathrm{CHO}>\mathrm{CH}_{2} \mathrm{ClCOCH}_{3}>\mathrm{CH}_{3} \mathrm{COCH}_{3}$


Q. Write the mechanism for the following conversion.


Ans.
 Phenylacetylene
Phenylacetylene
 Phenylacetylene
Phenylacetylene
Q. Carboxylic acids do not give the characteristic reactions of carbonyl group. Explain.
Ans. The carbonyl group in carboxylic acid is not a free group as in aldehydes and ketones. It is involved in resonance.
 Therefore, carboxylic acids fail to give the characteristic reactions of the carbonyl groups as given by aldehydes and ketones.
Therefore, carboxylic acids fail to give the characteristic reactions of the carbonyl groups as given by aldehydes and ketones.
 Therefore, carboxylic acids fail to give the characteristic reactions of the carbonyl groups as given by aldehydes and ketones.
Therefore, carboxylic acids fail to give the characteristic reactions of the carbonyl groups as given by aldehydes and ketones.
Q. Formic acid reduces Tollen’s reagent while other carboxylic acids do not. Justify.
Ans. $\mathrm{HCOOH}$ has an aldehydic (CHO) group in addition to
carboxyl group $(\mathrm{COOH}) .$ Therefore, it is expected to behave as reducing agent also and reduces Tollens to form a shining mirror.

Q. 2, 4, 6-trimethylbenzoic acid is quite difficult to esterify. Assign reason.
Ans.
 In the trimethylbenzoic acid there are three electron releasing $\mathrm{CH}_{3}$ groups present. They tend to increase the electron density on the oxygen atom of $O-H$ bond in carboxylic acid. As a result, the acidic strength is reduced considerably and it is not so easy to esterify $2,4,6$ -trimethyl benzoic acid.
In the trimethylbenzoic acid there are three electron releasing $\mathrm{CH}_{3}$ groups present. They tend to increase the electron density on the oxygen atom of $O-H$ bond in carboxylic acid. As a result, the acidic strength is reduced considerably and it is not so easy to esterify $2,4,6$ -trimethyl benzoic acid.
 In the trimethylbenzoic acid there are three electron releasing $\mathrm{CH}_{3}$ groups present. They tend to increase the electron density on the oxygen atom of $O-H$ bond in carboxylic acid. As a result, the acidic strength is reduced considerably and it is not so easy to esterify $2,4,6$ -trimethyl benzoic acid.
In the trimethylbenzoic acid there are three electron releasing $\mathrm{CH}_{3}$ groups present. They tend to increase the electron density on the oxygen atom of $O-H$ bond in carboxylic acid. As a result, the acidic strength is reduced considerably and it is not so easy to esterify $2,4,6$ -trimethyl benzoic acid.
Q. m-Hydroxybenzoic acid is a stronger acid than benzoic acid while p-hydroxybenzoic acid is weaker. Explain.
Ans. The hydroxyl $(O H)$ group has both $-I$ effect and $+R$ effect. Whereas the former helps in the release of $H^{+}$ from the carboxylic group, the latter tends to oppose the same because of resonance. At the para position, the $+\mathrm{R}$ effect dominates the effect. Therefore, p-hydroxy benzoic acid is a weaker acid than benzoic acid. But in case of m-hydroxybenzoic acid, the $+\mathrm{R}$ effect does not operate to the same extent as in para isomer. Thus, the acidic weakening effect is smaller and meta hydroxy benzoic acid is a stronger acid than benzoic acid.
Q. Carbon-oxygen bond lengths in formic acid are different but are the same in sodium formate. Justify.
Ans. In sodium formate, the contributing structures for the anion are equivalent while these are not the same in formic acid.
 Thus, carbon-oxygen bond length in formate ion is the same for both the bonds while these are different in formic acid.
Thus, carbon-oxygen bond length in formate ion is the same for both the bonds while these are different in formic acid.
 Thus, carbon-oxygen bond length in formate ion is the same for both the bonds while these are different in formic acid.
Thus, carbon-oxygen bond length in formate ion is the same for both the bonds while these are different in formic acid.
Q. Phenate ion has more number of contributing structures than benzoate ion; but still benzoic acid is a stronger acid. Explain.
Ans. In phenate ion, the negative charge available is dispersed only one electrongative oxygen atom while there are two oxygen atoms in benzoate ion to disperse the negative charge.
 This means that benzoate ion is more stable than phenate ion and this makes benzoic acid stronger acid than phenol although the contributing structures for phenate ion (five) are more than for benzoate ion (two).
This means that benzoate ion is more stable than phenate ion and this makes benzoic acid stronger acid than phenol although the contributing structures for phenate ion (five) are more than for benzoate ion (two).
 This means that benzoate ion is more stable than phenate ion and this makes benzoic acid stronger acid than phenol although the contributing structures for phenate ion (five) are more than for benzoate ion (two).
This means that benzoate ion is more stable than phenate ion and this makes benzoic acid stronger acid than phenol although the contributing structures for phenate ion (five) are more than for benzoate ion (two).
Q. Tertiary butyl benzene does not give benzoic acid when oxidised with $K M n O_{4} .$ Why?
Ans. In order that an alkyl group attached to benzene ring may be oxidised to carboxyl group, the presence of atleast one
$\alpha$ -hydrogen is necessary. For example, toluene is oxidised to benzoic acid. But tertiary butyl benzene has no -hydrogen atom. It is therefore, not oxidised to benzoic acid.




Q. $\mathrm{CH}_{3} \mathrm{COO}^{-}$ ion is more stable than $\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{O}^{-}$ ion. Assign reason.
Ans. In acetate ion, the negative charge gets dispersed due to the electron withdrawing nature of carbonyl group. On the other
hand, in ethoxide ion, the $C_{2} H_{5}$ group with $+I$ effect tends to increase the electron density on the ion and destabilise it.


Q. Which out of each pair is expected to be a stronger acid ?
(a) $\quad \mathrm{CH}_{3} \mathrm{COOH}$ or $\mathrm{HCOOH}$
(b) $\quad \mathrm{CH}_{3} \mathrm{COOH}$ or $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}$
(c) $\quad C_{6} H_{5} \mathrm{COOH}$ or $\mathrm{HCOOH}$
(d) $\quad C H_{2}(C l) C O O H$ or $C H_{2}(B r) C O O H$


Ans. (a) $\mathrm{HCOOH}$
(b) $\mathrm{CH}_{3} \mathrm{COOH}$
(c) HCOOH
(d) $\mathrm{CH}_{2}(\mathrm{Cl}) \mathrm{COOH}$


Q. How will you prepare 2-methylbutanoic acid from butan-2-ol ?
Ans.




Q. Complete following :


Ans.


Q. How will you synthesise the following by using a Grignard reagent?
(a) $2,2$ -Dimethylpentanoic acid (b) But-3-enoic acid
Ans.




Q. Why is acetyl chloride a better acetylating agent than acetic acid ?
Ans. The acetylation is carried by acetyl carbocation $\left(\mathrm{CH}_{3} \mathrm{CO}^{+}\right)$ The cation is formed by acetyl chloride and not by acetic acid.
 Therefore, acetyl chloride is a better acetylating agent than acetic acids.
Therefore, acetyl chloride is a better acetylating agent than acetic acids.
 Therefore, acetyl chloride is a better acetylating agent than acetic acids.
Therefore, acetyl chloride is a better acetylating agent than acetic acids.
Q. Draw the structural formula of hex-2-en-4-ynoic acid
 Describe the following giving a chemical equation for each
(i) Transesterification,
(ii) Hofmann bromamide reaction
Describe the following giving a chemical equation for each
(i) Transesterification,
(ii) Hofmann bromamide reaction
 Describe the following giving a chemical equation for each
(i) Transesterification,
(ii) Hofmann bromamide reaction
Describe the following giving a chemical equation for each
(i) Transesterification,
(ii) Hofmann bromamide reaction
Ans. (i) Transesterification : This reaction involves the nucleophilic acyl substitution of the alkoxy group of an ester with the alkoxy group of an alcohol in the presence of an acid or alkali
$R C O O R^{\prime}+R^{\prime \prime} O H \longrightarrow R C O O R^{\prime \prime}+R^{\prime} O H$
(ii) Hofmann bromamide reaction: This reaction involves the conversion of an amide into primary amine with one carbon atom less than the parent amide, by reaction with bromine and alkali


Q. Predict the products of the following reactions :


Ans.
 (c) $\quad \mathrm{NH}_{2}$ attached to $\mathrm{NH}$ is more nucleophile than $\mathrm{NH}_{2}$ attached to $\mathrm{C}=$ O group, therefore, reaction occurs through $\mathrm{NH}_{2}$ attached to NH to give the corresponding semicarbozone, i.e.,
(c) $\quad \mathrm{NH}_{2}$ attached to $\mathrm{NH}$ is more nucleophile than $\mathrm{NH}_{2}$ attached to $\mathrm{C}=$ O group, therefore, reaction occurs through $\mathrm{NH}_{2}$ attached to NH to give the corresponding semicarbozone, i.e.,

 (c) $\quad \mathrm{NH}_{2}$ attached to $\mathrm{NH}$ is more nucleophile than $\mathrm{NH}_{2}$ attached to $\mathrm{C}=$ O group, therefore, reaction occurs through $\mathrm{NH}_{2}$ attached to NH to give the corresponding semicarbozone, i.e.,
(c) $\quad \mathrm{NH}_{2}$ attached to $\mathrm{NH}$ is more nucleophile than $\mathrm{NH}_{2}$ attached to $\mathrm{C}=$ O group, therefore, reaction occurs through $\mathrm{NH}_{2}$ attached to NH to give the corresponding semicarbozone, i.e.,

Q. Which acid of each pair shown here would you expect to be stronger ?


Ans.








Q. Although phenoxide ion has more number of resonating structure than carboxylate ion, carboxylic acid is a stronger acid than phenol. Why?
Ans. In the resonating structure of phenoxide ion the negative charge is dispersed on only one oxygen atom whereas in the carboxylate ion the charge gets dispersed on two oxygen atoms. Thus, the dispersal of charge is more in the case of carboxylate ion and hence the resonance stabilisation is more. This is why the carboxylic acids are more stronger than phenol (s).
Q. Give reasons for the following :
(i) Aldehydes are more reactive than ketones in nucleophilic reactions.
(ii) Most aromatic acids are solids but the acids of acetic acid group are mostly liquids.
Ans. (i) The reactivities of aldehydes is more than that of ketones due to the following reasons:
(a) Inductive effect: The ease with which a nucleophile attacks the carbonyl group depends upon the electron deficiency i.e., the magnitude of the positive charge on carbonyl carbon. since an alkyl group has electron donating inductive effect ( $+$ I effect), therefore greater the number of alkyl groups attached to carbonyl carbon greater is the electron density on the carbonyl carbon and hence lower is its reactivity. Aldehydes are more polar than ketones.
 In ketone we have two alkyl groups whereas in aldehydes only one alkyl group is attached to carbonyl carbon. Since there is lesser number of alkyl groups in case of aldehydes so they undergo nucleophillic addition more readily than ketones.
(b) Steric effect : The presence of more alkyl groups in case of ketones hinders the attack of nucleophile on the carbonyl group and so the ketones are less reactive than aldehydes.
(ii) Aromatic acids contain benzene ring and their molecular masses are much higher than aliphatic acids of acetic acid group. Aromatic, acids also involve stronger inter molecular attractions, thus they are solids.
In ketone we have two alkyl groups whereas in aldehydes only one alkyl group is attached to carbonyl carbon. Since there is lesser number of alkyl groups in case of aldehydes so they undergo nucleophillic addition more readily than ketones.
(b) Steric effect : The presence of more alkyl groups in case of ketones hinders the attack of nucleophile on the carbonyl group and so the ketones are less reactive than aldehydes.
(ii) Aromatic acids contain benzene ring and their molecular masses are much higher than aliphatic acids of acetic acid group. Aromatic, acids also involve stronger inter molecular attractions, thus they are solids.
 In ketone we have two alkyl groups whereas in aldehydes only one alkyl group is attached to carbonyl carbon. Since there is lesser number of alkyl groups in case of aldehydes so they undergo nucleophillic addition more readily than ketones.
(b) Steric effect : The presence of more alkyl groups in case of ketones hinders the attack of nucleophile on the carbonyl group and so the ketones are less reactive than aldehydes.
(ii) Aromatic acids contain benzene ring and their molecular masses are much higher than aliphatic acids of acetic acid group. Aromatic, acids also involve stronger inter molecular attractions, thus they are solids.
In ketone we have two alkyl groups whereas in aldehydes only one alkyl group is attached to carbonyl carbon. Since there is lesser number of alkyl groups in case of aldehydes so they undergo nucleophillic addition more readily than ketones.
(b) Steric effect : The presence of more alkyl groups in case of ketones hinders the attack of nucleophile on the carbonyl group and so the ketones are less reactive than aldehydes.
(ii) Aromatic acids contain benzene ring and their molecular masses are much higher than aliphatic acids of acetic acid group. Aromatic, acids also involve stronger inter molecular attractions, thus they are solids.
Q. Write IUPAC names of following compounds :


Ans.
 $2-(2-$ bromophenyl) ethanal. It can also be named as
$2-(\text { o-bromophenyl })$ ethanal.
(b) 5 -chloro-3-ethyl-2-pentanone.
(c) $3,5$ -dimethyl phenyl ethanoate
$2-(2-$ bromophenyl) ethanal. It can also be named as
$2-(\text { o-bromophenyl })$ ethanal.
(b) 5 -chloro-3-ethyl-2-pentanone.
(c) $3,5$ -dimethyl phenyl ethanoate
 $2-(2-$ bromophenyl) ethanal. It can also be named as
$2-(\text { o-bromophenyl })$ ethanal.
(b) 5 -chloro-3-ethyl-2-pentanone.
(c) $3,5$ -dimethyl phenyl ethanoate
$2-(2-$ bromophenyl) ethanal. It can also be named as
$2-(\text { o-bromophenyl })$ ethanal.
(b) 5 -chloro-3-ethyl-2-pentanone.
(c) $3,5$ -dimethyl phenyl ethanoate
Q. Give reasons for the following :
(a) Acetic acid is a weaker acid than chloroacetic acid.
(b) During preparation of ammonia derivatives of aldehydes and ketones, $p H$ of medium is controlled.
Ans. (a) The acidic nature of a carboxylic acid is due to its ability to release the $H^{+}$ (proton). Any factor that stabilise the carboxylate ion would facilitate the release of proton and thus increase the acid strength. The electron withdrawing group (attached to $-\mathrm{COOH}$ gp) increases the acid strength. Due to electron withdrawing effect $(-I \text { effect })$ of chlorine atom, the negative charge of carboxylate ion formed gets dispersed. As such this ion gets stabilised and is, therefore, more easily formed.
 The acetate ion obtained from acetic acid gets detabilised due to electron releasing effect of methyl group and is not formed
easily. Consequently dissociation of $\mathrm{CH}_{3} \mathrm{COOH}$ takes place to lesser extent.
The acetate ion obtained from acetic acid gets detabilised due to electron releasing effect of methyl group and is not formed
easily. Consequently dissociation of $\mathrm{CH}_{3} \mathrm{COOH}$ takes place to lesser extent.
 (b) Formation of ammonia derivatives (oximes, hydrazone, semi-carbazone etc.) proceeds via the attack of carbonyl carbon with proton to form the conjugate acid.
(b) Formation of ammonia derivatives (oximes, hydrazone, semi-carbazone etc.) proceeds via the attack of carbonyl carbon with proton to form the conjugate acid.
 Therefore, presence of an acid is a must for preparing these
derivatives $(p H<7)$ However, in strongly acid medium, the proton attacks the unshared pair of electrons on nitrogen to form the species
$R N^{+} H_{3}$ which cannot attack the carbonyl carbon.
$\stackrel{+}{H}+: N H_{2} R \longrightarrow N H_{3} R$
In basic medium, there is no protonation of carbonyl group and
hence no reaction.
$>C=O \frac{\text { basic medium }}{\text { medium }} \rightarrow$ No protonation Therefore, preparation of ammonia derivatives requires slightly
acidic medium $(p H=3.5)$ and its careful control is essential.
Therefore, presence of an acid is a must for preparing these
derivatives $(p H<7)$ However, in strongly acid medium, the proton attacks the unshared pair of electrons on nitrogen to form the species
$R N^{+} H_{3}$ which cannot attack the carbonyl carbon.
$\stackrel{+}{H}+: N H_{2} R \longrightarrow N H_{3} R$
In basic medium, there is no protonation of carbonyl group and
hence no reaction.
$>C=O \frac{\text { basic medium }}{\text { medium }} \rightarrow$ No protonation Therefore, preparation of ammonia derivatives requires slightly
acidic medium $(p H=3.5)$ and its careful control is essential.
 The acetate ion obtained from acetic acid gets detabilised due to electron releasing effect of methyl group and is not formed
easily. Consequently dissociation of $\mathrm{CH}_{3} \mathrm{COOH}$ takes place to lesser extent.
The acetate ion obtained from acetic acid gets detabilised due to electron releasing effect of methyl group and is not formed
easily. Consequently dissociation of $\mathrm{CH}_{3} \mathrm{COOH}$ takes place to lesser extent.
 (b) Formation of ammonia derivatives (oximes, hydrazone, semi-carbazone etc.) proceeds via the attack of carbonyl carbon with proton to form the conjugate acid.
(b) Formation of ammonia derivatives (oximes, hydrazone, semi-carbazone etc.) proceeds via the attack of carbonyl carbon with proton to form the conjugate acid.
Q. How does $N H_{3}$ react with
(a) Formaldehyde,
(b) Acetaldehyde
(c) Semicarbazide
Ans. (a) $N H_{3}$ reacts with formaldehyde to form hexamethylene tetramine (also called urotropine).




Q. (a) What is ammonolysis of esters?
(b) How will you convert:
(i) Acetaldehyde to acetamide
(ii) Methanol to acetic acid?
Ans. (a) Esters react with ammonia to form amides. The reaction is called ammonolysis of esters.


Q. Addition of Grignard reagents to dry ice followed by hydrolysis gives carboxylic acids while that of organolithium compounds under similar conditions gives ketones. Explain
Ans. since electronegativity of $L i(\mathrm{E} . \mathrm{N} .=1.0)$ is lower than that of $M g(\mathrm{E.N.}=1.2),$ therefore, organolithium compounds are more nucleophilite than Grignard reagents. As a result, organolithium compounds not only add to the more reactive $\mathrm{CO}_{2}$ but also to the less reactive resonance stabilized lithium salt of carboxylic acid thus formed to yield ketones.
 Grignard reagents, on the other hand, being less nucleophilic
add only to the more reactive $\mathrm{CO}_{2}$ but not to the less reactive resonance stabilized magnesium salt of the carboxylic acid from which carbxylic acid can be generated by hydrolysis with mineral acids.
Grignard reagents, on the other hand, being less nucleophilic
add only to the more reactive $\mathrm{CO}_{2}$ but not to the less reactive resonance stabilized magnesium salt of the carboxylic acid from which carbxylic acid can be generated by hydrolysis with mineral acids.

 Grignard reagents, on the other hand, being less nucleophilic
add only to the more reactive $\mathrm{CO}_{2}$ but not to the less reactive resonance stabilized magnesium salt of the carboxylic acid from which carbxylic acid can be generated by hydrolysis with mineral acids.
Grignard reagents, on the other hand, being less nucleophilic
add only to the more reactive $\mathrm{CO}_{2}$ but not to the less reactive resonance stabilized magnesium salt of the carboxylic acid from which carbxylic acid can be generated by hydrolysis with mineral acids.

Q. How will you prepare methanol from formaldehyde without using a reducing agent.
Ans. Cannizzaro reaction is a disproportionation reaction in which one molecule of an aldehyde is reduced while the other is oxidised.
 Here a conc. solution of $N a O H$ is used which is not a reducing agent.
Here a conc. solution of $N a O H$ is used which is not a reducing agent.
 Here a conc. solution of $N a O H$ is used which is not a reducing agent.
Here a conc. solution of $N a O H$ is used which is not a reducing agent.
Q. You are provided with following four reagents. $I_{2} / N a O H$ $\mathrm{NaHSO}_{3},$ LiAlH_{4} Schiff's reagent. Write which two reagents can be used to distinguish between the compounds in each of the following pairs:
(i) $\quad \mathrm{CH}_{3} \mathrm{CHO}$ and $\mathrm{CH}_{3} \mathrm{COCH}_{3}$
(ii) $\quad \mathrm{CH}_{3} \mathrm{CHO}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}$
(iii) $\quad C_{6} H_{5} C O C H_{3}$ and $C_{6} H_{5} C O C_{6} H_{5}$
Ans. (i) $\quad \mathrm{CH}_{3} \mathrm{CHO}$ and $\mathrm{CH}_{3} \mathrm{COCH}_{3}$ can be distinguished by :
LiAIH$_{4}$ and Schiff's reagent.
(ii) $\mathrm{CH}_{3} \mathrm{CHO}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}$ can be distinguished by $\mathrm{I}_{2} / \mathrm{NaOH}$ and Schiff's reagent (Benzaldehy de restores pink colour to Schiff's reagent very slowly).
(iii) $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COCH}_{3}$ and $\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COC}_{6} \mathrm{H}_{5}$ can be distinguished by
$\mathrm{NaHSO}_{3}$ and $\mathrm{I}_{2} / \mathrm{NaOH}$
Q. An organic compound [A] molecular formula $C_{4} H_{8} O$ when reduced with $\mathrm{NaBH}_{4}$ gives compound [\textrm{B} ] \text { which reacts with } HBr to form compound [C][\mathrm{ optically active } ] \text { Identify } \mathrm { A } , \mathrm { B } , \mathrm { C }
and write the two enantiomers of compound $C$.
Ans. The data suggests that the given compound [A] is an aliphatic
ketone butanone $\left(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{COCH}_{3}\right) .$ The reactions involved are listed.

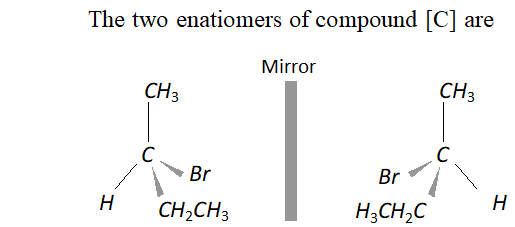


Q. Two moles of an ester $[\mathrm{A}]$ are condensed in the presence of sodium ethoxide to give a $\beta$ -ketoester $[\mathrm{B}]$ and ethanol. On heating in an acidic solution $[\mathrm{B}]$ gives ethanol and a $\beta$ -ketoacid $[\mathrm{C}] .$ On decarboxylation, [C] gives pentan- 3 -one. Identify $[\mathrm{A}],[\mathrm{B}]$ and $[\mathrm{C}]$ with proper reasoning.
Ans. (a) According to available data [C] is a $\beta$ -keto acid which upon decarboxylation gives pentane- 3 -one. The structure of is :
$[\mathrm{C}]$
 (b) The compound $[\mathrm{C}]$ along with ethanol has been obtained from compound [B] upon acid hydrolysis. since $[\mathrm{B}]$ is a $\beta$ ketoester, its structure may be given as follows:
(b) The compound $[\mathrm{C}]$ along with ethanol has been obtained from compound [B] upon acid hydrolysis. since $[\mathrm{B}]$ is a $\beta$ ketoester, its structure may be given as follows:
 (c) since $[\mathrm{B}]$ has been formed by the condensation of two moles of $[\mathrm{A}]$ in the presence of sodium ethoxide, the ester $[\mathrm{A}]$ is ethyl propanoate and it participates in Claisen Condensation as follows:
(c) since $[\mathrm{B}]$ has been formed by the condensation of two moles of $[\mathrm{A}]$ in the presence of sodium ethoxide, the ester $[\mathrm{A}]$ is ethyl propanoate and it participates in Claisen Condensation as follows:


 (b) The compound $[\mathrm{C}]$ along with ethanol has been obtained from compound [B] upon acid hydrolysis. since $[\mathrm{B}]$ is a $\beta$ ketoester, its structure may be given as follows:
(b) The compound $[\mathrm{C}]$ along with ethanol has been obtained from compound [B] upon acid hydrolysis. since $[\mathrm{B}]$ is a $\beta$ ketoester, its structure may be given as follows:
 (c) since $[\mathrm{B}]$ has been formed by the condensation of two moles of $[\mathrm{A}]$ in the presence of sodium ethoxide, the ester $[\mathrm{A}]$ is ethyl propanoate and it participates in Claisen Condensation as follows:
(c) since $[\mathrm{B}]$ has been formed by the condensation of two moles of $[\mathrm{A}]$ in the presence of sodium ethoxide, the ester $[\mathrm{A}]$ is ethyl propanoate and it participates in Claisen Condensation as follows:


Q. Give chemical tests to distinguish between the following pairs of compounds :
(a) Propanal and Propanone
(b) Methyl acetate and ethyl acetate
(c) Benzaldehyde and Benzoic acid
Ans. (a) When propanal is added to tollen's reagent (an ammoniacal solution of silver nitrate), silver oxide is reduced to metallic silver which deposits as a mirre
 (b) When ethyl acetate is treated with aqueous $N a O H$ Sodium ethanoate and ethanol is formed ethanol on treatment with $I_{2}$ and $N a O H$ forms yellow ppt of iodoform.
(b) When ethyl acetate is treated with aqueous $N a O H$ Sodium ethanoate and ethanol is formed ethanol on treatment with $I_{2}$ and $N a O H$ forms yellow ppt of iodoform.
 When methyl acetate is treated with aqueous NaOH sodium ethanoate and Methanol is formed. Methanol does not respond to iodoform reaction
When methyl acetate is treated with aqueous NaOH sodium ethanoate and Methanol is formed. Methanol does not respond to iodoform reaction
 (c) When benzoic acid is warmed with ethyl alcohol and a little concentrated $\mathrm{H}_{2} \mathrm{SO}_{4},$ a fragrant odour of ethyl benzoate is obtained.
(c) When benzoic acid is warmed with ethyl alcohol and a little concentrated $\mathrm{H}_{2} \mathrm{SO}_{4},$ a fragrant odour of ethyl benzoate is obtained.
 Benzaldehyde does not respond to this test.
Benzaldehyde does not respond to this test.
 (b) When ethyl acetate is treated with aqueous $N a O H$ Sodium ethanoate and ethanol is formed ethanol on treatment with $I_{2}$ and $N a O H$ forms yellow ppt of iodoform.
(b) When ethyl acetate is treated with aqueous $N a O H$ Sodium ethanoate and ethanol is formed ethanol on treatment with $I_{2}$ and $N a O H$ forms yellow ppt of iodoform.
 When methyl acetate is treated with aqueous NaOH sodium ethanoate and Methanol is formed. Methanol does not respond to iodoform reaction
When methyl acetate is treated with aqueous NaOH sodium ethanoate and Methanol is formed. Methanol does not respond to iodoform reaction
 (c) When benzoic acid is warmed with ethyl alcohol and a little concentrated $\mathrm{H}_{2} \mathrm{SO}_{4},$ a fragrant odour of ethyl benzoate is obtained.
(c) When benzoic acid is warmed with ethyl alcohol and a little concentrated $\mathrm{H}_{2} \mathrm{SO}_{4},$ a fragrant odour of ethyl benzoate is obtained.
Q. Write structural formulae and names of the four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde served as nucleophile and which as electrophile.
Ans.




Q. An organic compound (A) (molecular formula $\left.C_{8} H_{16} O_{2}\right)$ was hydrolysed with dilute sulphuric acid to give a carboxylic acid
(B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). (C) on dehydration gives but-1-rene. Write equations for the reactions involved.
Ans.
 Dehydration of C gives but-1-ene i.e., a product with four carbon atoms, therefore the other product B is also with four carbon atoms and has a terminal COOH group.
Accordingly, A is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOCH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}$
(butyl butanoate)
$\mathrm{B}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$ and
$\mathrm{C}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{OH}$
Dehydration of C gives but-1-ene i.e., a product with four carbon atoms, therefore the other product B is also with four carbon atoms and has a terminal COOH group.
Accordingly, A is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOCH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}$
(butyl butanoate)
$\mathrm{B}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$ and
$\mathrm{C}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{OH}$
 Dehydration of C gives but-1-ene i.e., a product with four carbon atoms, therefore the other product B is also with four carbon atoms and has a terminal COOH group.
Accordingly, A is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOCH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}$
(butyl butanoate)
$\mathrm{B}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$ and
$\mathrm{C}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{OH}$
Dehydration of C gives but-1-ene i.e., a product with four carbon atoms, therefore the other product B is also with four carbon atoms and has a terminal COOH group.
Accordingly, A is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOCH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}$
(butyl butanoate)
$\mathrm{B}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$ and
$\mathrm{C}$ is $\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{OH}$
Q. Arrange the following compounds in increasing order of their property as indicated:
(a) Acetaldehyde, Acetone, Di-tert-butyl ketone, methyl tertbutyl ketone (reactivity towards $H C N)$
(b) $C H_{3} C H_{2} C H(B r) \operatorname{COOH}, C H_{3} C H(B r) C H_{2} C O O H$
$\left(\mathrm{CH}_{3}\right) \mathrm{CHCOOH}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$ (acid strength)
(c) Benzoic acid, 4-Nitrobenzoic acid, 3, 4- Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)
Ans. (a) The reactivity of aldehydes and ketones toward nudeophillic addition depends upon (i) $+$ I effect (ii) steric hinderance. Hence the order is Di-tert-butyl ketone $<$ methyl-tert-butyl ketone < Acetone $<$ Acetaldehy de.
(b) The acidic strength depends upon (i) nature of $+$ I effect
(ii) nature of atom/group attached (iii) position of substituent on the chain. Hence, $\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHCOOH}<\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{COOH}$
$<\mathrm{CH}_{3} \mathrm{CH}(\mathrm{Br}) \mathrm{CH}_{2} \mathrm{COOH}<\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}(\mathrm{Br}) \mathrm{COOH}$
(c) $\quad 4$ -Methoxy benzoic acid $<$ Benzoic acid $<4$ Nitrobenzoic acid $<3,4$ -Dinitrobenzoic acid
Q. Give plausibe explanation for each of the following
(a) Cyclohexanone forms cyanohydrin in good yield but 2 ,
$2,6$ -trimethylcyclohexanone does not.
(b) There are two $-N H_{2}$ groups in semicarbazide. However, only one is involved in the formation of semicarbazones.
(c) During the preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, the
water or the ester should be removed as fast as it is formed.
Ans. (a) The carbonyl group in cyclohexanone is highly
polarised and nuleophilic addition of $H^{\delta+}-C N^{\delta-}$ at carboxyl
group $\left(>C^{\delta+}=O^{\delta-}\right)$ takes place easily. centre of nucleophilic attack by $C N^{-}$
 The presence of three methyl group (which are electron repelling) reduces the polarity of $>C=O$ group on one hand and offer a steric hindrance to nucleophilic attack of $C N^{-}$ at group. Therefore, trimethyl cyclohexanone does not give good yield i.e., it gives very poor yield.
(b) The study of semicarbazide (NH3 derivative of urea,
The presence of three methyl group (which are electron repelling) reduces the polarity of $>C=O$ group on one hand and offer a steric hindrance to nucleophilic attack of $C N^{-}$ at group. Therefore, trimethyl cyclohexanone does not give good yield i.e., it gives very poor yield.
(b) The study of semicarbazide (NH3 derivative of urea,
 reveal that two $\mathrm{N}-\mathrm{H}$ bonds of $\mathrm{NH}_{2}$ group away from group in semicarbazide are comparatively weaker than two $\mathrm{N}-\mathrm{H}$ bond of the other $-N H_{2}$ group closer to $>C=O$ group. Thus it
produces $H^{+}$ easily for protonation of group of aldehyde or ketone.
reveal that two $\mathrm{N}-\mathrm{H}$ bonds of $\mathrm{NH}_{2}$ group away from group in semicarbazide are comparatively weaker than two $\mathrm{N}-\mathrm{H}$ bond of the other $-N H_{2}$ group closer to $>C=O$ group. Thus it
produces $H^{+}$ easily for protonation of group of aldehyde or ketone.

 The nature of reaction, esterification of carboxylic acid, is reversible as ester formed react with water and produces reactants and thus an equilibrium is set up between reactants and products. The removal of ester or water shifts the equilibrium to right according to Le-Chatelier's principle and more ester is formed.
The nature of reaction, esterification of carboxylic acid, is reversible as ester formed react with water and produces reactants and thus an equilibrium is set up between reactants and products. The removal of ester or water shifts the equilibrium to right according to Le-Chatelier's principle and more ester is formed.
 The presence of three methyl group (which are electron repelling) reduces the polarity of $>C=O$ group on one hand and offer a steric hindrance to nucleophilic attack of $C N^{-}$ at group. Therefore, trimethyl cyclohexanone does not give good yield i.e., it gives very poor yield.
(b) The study of semicarbazide (NH3 derivative of urea,
The presence of three methyl group (which are electron repelling) reduces the polarity of $>C=O$ group on one hand and offer a steric hindrance to nucleophilic attack of $C N^{-}$ at group. Therefore, trimethyl cyclohexanone does not give good yield i.e., it gives very poor yield.
(b) The study of semicarbazide (NH3 derivative of urea,

 The nature of reaction, esterification of carboxylic acid, is reversible as ester formed react with water and produces reactants and thus an equilibrium is set up between reactants and products. The removal of ester or water shifts the equilibrium to right according to Le-Chatelier's principle and more ester is formed.
The nature of reaction, esterification of carboxylic acid, is reversible as ester formed react with water and produces reactants and thus an equilibrium is set up between reactants and products. The removal of ester or water shifts the equilibrium to right according to Le-Chatelier's principle and more ester is formed.
Q. Predict the organic product of the following reactions




Ans.




Q. (a) How will you convert
(i) Benzoyl chloride to benzaldehyde
(ii) Propanone to 2 -propanol
(iii) Benzoic acid to $m$ -nitrobenzoic acid
(Write the reaction and state the reaction conditions in each case.)
(b) Write the names and structures of the products formed in the following reactions
(i) Reaction of semicarbazide $\left(N H_{2} C O N H N H_{2}\right)$ with formaldehyde.
(ii) Oxidation of ethyl benzene with alkaline $K M n O_{4}$.
Ans. (a) (i) Benzoyl chloride is subjected to Rosenmund reduction.




Q. Account for the following
(i) Chloroacetic acid has lower $p K a$ value than acetic acid
(ii) Electrophillic substitution in benzoic acid takes place at meta position.
(iii) Carboxylic acid have higher boiling points than alcohols of comparable molecular masses.
Ans. (i) Chloroacetic acid is a stronger acid than acetic acid and has higher value of dissociation constant $K a$ than that of acetic acid. We know that $p K a=-\log K a,$ it means that chloroacetic acid having higher value $K a$ will have lower value of $p K a .$ Chloroacetic acid is stronger than acetic acid because $C l$ group is electron withdrawing and chloroacetate ion is more stabilised than acetate ion (as methyl group is electron releasing).
 (ii) Carboxyl group (-COOH) is electron withdrawing i.e. deactivating the benzene ring and thus electron density becomes very less at ortho and para position in comparison to meta position. Electrophiles (+vely charged species) find it easier to attack at meta position as there is higher electron density thus $-C O O H$ group is meta directing.
(ii) Carboxyl group (-COOH) is electron withdrawing i.e. deactivating the benzene ring and thus electron density becomes very less at ortho and para position in comparison to meta position. Electrophiles (+vely charged species) find it easier to attack at meta position as there is higher electron density thus $-C O O H$ group is meta directing.
 As there is positive charge on ortho and para position, electron density is higher at meta position and hence electrophilic substitution takes place at meta position. (Similarly nitro group is meta directing)
(iii) Carboxylic group (-COOH) in acids is highly polar and carboxylic acid, generally, exists as dimers containing two hydrogen bonds each as shown below.
As there is positive charge on ortho and para position, electron density is higher at meta position and hence electrophilic substitution takes place at meta position. (Similarly nitro group is meta directing)
(iii) Carboxylic group (-COOH) in acids is highly polar and carboxylic acid, generally, exists as dimers containing two hydrogen bonds each as shown below.
 These hydrogen bonds in carboxylic acids are stronger than those in alcohols. It is due to following two factors
(a) The $O-H$ bond of the carboxylic acids are more strongly polarised due to adjacent electron attracting $>C=$ O groups.
(b) The oxygen atom of the group $C=O$ in carboxylic acid is more negative as compared to the oxygen atom of the alcohol.
These hydrogen bonds in carboxylic acids are stronger than those in alcohols. It is due to following two factors
(a) The $O-H$ bond of the carboxylic acids are more strongly polarised due to adjacent electron attracting $>C=$ O groups.
(b) The oxygen atom of the group $C=O$ in carboxylic acid is more negative as compared to the oxygen atom of the alcohol.
 Thus carboxylic acids posses higher boiling points than corresponding alcohols of similar molecular masses.
Thus carboxylic acids posses higher boiling points than corresponding alcohols of similar molecular masses.
 (ii) Carboxyl group (-COOH) is electron withdrawing i.e. deactivating the benzene ring and thus electron density becomes very less at ortho and para position in comparison to meta position. Electrophiles (+vely charged species) find it easier to attack at meta position as there is higher electron density thus $-C O O H$ group is meta directing.
(ii) Carboxyl group (-COOH) is electron withdrawing i.e. deactivating the benzene ring and thus electron density becomes very less at ortho and para position in comparison to meta position. Electrophiles (+vely charged species) find it easier to attack at meta position as there is higher electron density thus $-C O O H$ group is meta directing.
 As there is positive charge on ortho and para position, electron density is higher at meta position and hence electrophilic substitution takes place at meta position. (Similarly nitro group is meta directing)
(iii) Carboxylic group (-COOH) in acids is highly polar and carboxylic acid, generally, exists as dimers containing two hydrogen bonds each as shown below.
As there is positive charge on ortho and para position, electron density is higher at meta position and hence electrophilic substitution takes place at meta position. (Similarly nitro group is meta directing)
(iii) Carboxylic group (-COOH) in acids is highly polar and carboxylic acid, generally, exists as dimers containing two hydrogen bonds each as shown below.
 These hydrogen bonds in carboxylic acids are stronger than those in alcohols. It is due to following two factors
(a) The $O-H$ bond of the carboxylic acids are more strongly polarised due to adjacent electron attracting $>C=$ O groups.
(b) The oxygen atom of the group $C=O$ in carboxylic acid is more negative as compared to the oxygen atom of the alcohol.
These hydrogen bonds in carboxylic acids are stronger than those in alcohols. It is due to following two factors
(a) The $O-H$ bond of the carboxylic acids are more strongly polarised due to adjacent electron attracting $>C=$ O groups.
(b) The oxygen atom of the group $C=O$ in carboxylic acid is more negative as compared to the oxygen atom of the alcohol.
 Thus carboxylic acids posses higher boiling points than corresponding alcohols of similar molecular masses.
Thus carboxylic acids posses higher boiling points than corresponding alcohols of similar molecular masses.
Q. Write chemical reactions for preparation of acetone from the following
(i) $\quad$ A secondary alcohol
(ii) Carboxylic acid
(iii) Acid chloride
(iv) Alkyne
(v) Alkene
Ans. (i) Acetone can be prepared by oxidation or catalytic dehydrogenation of isopropyl alcohol.






Q. How will you convert.
(a) Ethanal to lactic acid
(b) Acetaldehyde to acetone
(c) Ethanal to 2 -hydroxybut- 3 -enoic acid
(d) Formaldehyde to acetaldehyde
(e) Acetaldehyde to formaldehyde
(f) Formaldehyde to $n$ -butane
(g) Ethanol to butan- 2 -one
(h) Acetaldehyde to crotonic acid
(i) Propanal to propyne
(j) Acetone to phorone
(k) Propanone to iodoform
(1) Formaldehyde to urotropine
(m) Acetone to tertiary butyl alcohol
(n) Ethanal to propan- 2 -ol
(o) Methyl cyanide to ethanal
(p) Benzalchloride into cinnamaldehyde.
(q) Propene to propanone
(r) Acetophenone to 2 -phenylbutan- 2 -ol
Ans. (a) Ethanal to lactic acid



















Q. Write the equations for the reaction of :
 reagents:
(i) Heating with excess of $H B r$
(ii) Dilute $K M n O_{4}$ in the presence of alkali
(iii) A dilute solution of $N a_{2} C O_{3}$
(iv) Phenyl hydrazine
(v) Tollen's reagent
reagents:
(i) Heating with excess of $H B r$
(ii) Dilute $K M n O_{4}$ in the presence of alkali
(iii) A dilute solution of $N a_{2} C O_{3}$
(iv) Phenyl hydrazine
(v) Tollen's reagent
Ans.






Q. An organic compound (A) with molecular formula $\mathrm{C}_{8} \mathrm{H}_{8} \mathrm{O}$ forms an orange-red precipitate with $2,4$ -DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide. It neither reduces Tollen's or Fehlings' reagent, nor does it decolourise bromine water or Baeyer's reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula $C_{7} H_{6} O_{2} .$ Identify the compounds $(\mathrm{A})$ and $(\mathrm{B})$ and explain the reactions involved.
Ans. (A) forms $2,4$ -DNP derivative. Therefore, it is an aldehyde or a ketone. since it does not reduce Tollen's or Fehling reagent,
(A) must be a ketone. (A) responds to iodoform test. Therefore, it should be a methyl ketone. The molecular formula of (A) indicates high degree of unsaturation, yet it does not decolourise bromine water or Baeyer's reagent. This indicates the presence of unsaturation due to an aromatic ring. Compound (B), being an oxidation product of a ketone should be a carboxylic acid. The molecular formula of (B) indicates that it should be benzoic acid and compound (A) should, therefore, be a monosubstituted aromatic methyl ketone. The molecular formula of $(\mathrm{A})$ indicates that it should be phenyl methyl ketone (acetophenone). Reactions are as follows:




Q. What is meant by the following terms? Give an example in each case.
(a) Cyanohydrin
(b) Semicarbazone
(c) Hemiacetal
(d) Ketal
(e) $2,4$ -DNP-derivative
(f) Acetal
(g) Aldol
(h) Oxime
(i) Imine
(j) Schiff's base
Ans. (a) Cyanohydrin is a compound which contains both OH and CN group. For example, Lactic acid can be obtained by hydrolysis of cyanohydrin.
 (b) The product of carbonyl compounds with semicarbazide is known as semicarbazone.
(b) The product of carbonyl compounds with semicarbazide is known as semicarbazone.
 (c) Hemiacetal is a compound which contains an ether as well as alcohol functional group. For example, methoxyethanol is a hemiacetal.
(c) Hemiacetal is a compound which contains an ether as well as alcohol functional group. For example, methoxyethanol is a hemiacetal.
 (d) Ketal is a cyclic compound obtained by reaction of acetone with ethylene glycol.
(d) Ketal is a cyclic compound obtained by reaction of acetone with ethylene glycol.
 (e) $2,4$ -DNP derivative: see properties of aldehydes/ ketones in section 12.7 of text.
(f) Acetal : Acetal are the product of aldehyde and monohydric alcohol
(e) $2,4$ -DNP derivative: see properties of aldehydes/ ketones in section 12.7 of text.
(f) Acetal : Acetal are the product of aldehyde and monohydric alcohol
 (g) Aldol is a condensation product of acetaldehyde in the presence of dil. $N a O H$
(g) Aldol is a condensation product of acetaldehyde in the presence of dil. $N a O H$
 (h) Oxime : Aldehyde and Ketones react with hydroxylamine to form oximes.
(h) Oxime : Aldehyde and Ketones react with hydroxylamine to form oximes.
 (i) Schiff's base : Schiff's reagent is an aqueous solution of magenta or pink coloured rosaniline hydrochloride which has been decolourised by passing $\mathrm{SO}_{2}$ when aldehyde are treated with decolourised solution of schiff's reagent, its pink or magenta colour is restored. This reaction is used as a test for aldehyde because ketones do not restore the pink colour of schiff reagent.
(i) Schiff's base : Schiff's reagent is an aqueous solution of magenta or pink coloured rosaniline hydrochloride which has been decolourised by passing $\mathrm{SO}_{2}$ when aldehyde are treated with decolourised solution of schiff's reagent, its pink or magenta colour is restored. This reaction is used as a test for aldehyde because ketones do not restore the pink colour of schiff reagent.
 (b) The product of carbonyl compounds with semicarbazide is known as semicarbazone.
(b) The product of carbonyl compounds with semicarbazide is known as semicarbazone.
 (c) Hemiacetal is a compound which contains an ether as well as alcohol functional group. For example, methoxyethanol is a hemiacetal.
(c) Hemiacetal is a compound which contains an ether as well as alcohol functional group. For example, methoxyethanol is a hemiacetal.
 (d) Ketal is a cyclic compound obtained by reaction of acetone with ethylene glycol.
(d) Ketal is a cyclic compound obtained by reaction of acetone with ethylene glycol.
 (e) $2,4$ -DNP derivative: see properties of aldehydes/ ketones in section 12.7 of text.
(f) Acetal : Acetal are the product of aldehyde and monohydric alcohol
(e) $2,4$ -DNP derivative: see properties of aldehydes/ ketones in section 12.7 of text.
(f) Acetal : Acetal are the product of aldehyde and monohydric alcohol
 (g) Aldol is a condensation product of acetaldehyde in the presence of dil. $N a O H$
(g) Aldol is a condensation product of acetaldehyde in the presence of dil. $N a O H$
 (h) Oxime : Aldehyde and Ketones react with hydroxylamine to form oximes.
(h) Oxime : Aldehyde and Ketones react with hydroxylamine to form oximes.
 (i) Schiff's base : Schiff's reagent is an aqueous solution of magenta or pink coloured rosaniline hydrochloride which has been decolourised by passing $\mathrm{SO}_{2}$ when aldehyde are treated with decolourised solution of schiff's reagent, its pink or magenta colour is restored. This reaction is used as a test for aldehyde because ketones do not restore the pink colour of schiff reagent.
(i) Schiff's base : Schiff's reagent is an aqueous solution of magenta or pink coloured rosaniline hydrochloride which has been decolourised by passing $\mathrm{SO}_{2}$ when aldehyde are treated with decolourised solution of schiff's reagent, its pink or magenta colour is restored. This reaction is used as a test for aldehyde because ketones do not restore the pink colour of schiff reagent.
